Challenges of analytical method validation for ATMPs
pharmaphorum
JUNE 22, 2023
Challenges of analytical method validation for ATMPs Mike.Hammerton Thu, 22/06/2023 - 08:00 Bookmark this
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 Method Validation Related Topics
Method Validation Related Topics 
pharmaphorum
JUNE 22, 2023
Challenges of analytical method validation for ATMPs Mike.Hammerton Thu, 22/06/2023 - 08:00 Bookmark this

PharmaTech
FEBRUARY 2, 2023
Schniepp, distinguished fellow for Regulatory Compliance Associates, and Shiri Hechter, senior lab operations manager for Nelson Laboratories, provide a simple approach to validating analytical methods.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharma Pathway
JANUARY 24, 2023
Walk-In Interview for FR&D- OSD/ AR&D -OSD & Injectable/AR&D- Method Validations/ DQA -Analytical DQA Department: FR&D- OSD/ AR&D -OSD & Injectable/AR&D- Method Validations/ DQA -Analytical DQA Qualification: M.Pharm/ Analysis / M.Sc Dear, Greetings from Hetero…!!

Pharma Pathway
JANUARY 26, 2023
Sampling of FG/RM/PM Analytical Method Validation & Calibration Date of Interview: 29th Jan’ 2023 Interview Time: 09:00 AM to 16:00 PM Venue Details: Rang Lords Inn Nr.

European Pharmaceutical Review
APRIL 2, 2024
Timely implementation of the reference to USP <86> in the water for injection and purified water monographs is desired to encourage and enable the adoption of these methods for environmental monitoring purposes. Want to learn more about endotoxin testing and validation…?

Pharma Pathway
JANUARY 26, 2023
Sampling of FG/RM/PM Analytical Method Validation & Calibration Date of Interview: 29th Jan’ 2023 Interview Time: 09:00 AM to 16:00 PM Venue Details: Rang Lords Inn Nr.

Pharma Pathway
JANUARY 27, 2023
Experience: 01 to 04 years Department: AR&D- Method Validation API (Males) Qualification: M.Sc/ M. Pharmacy (Analytical Chemistry) Experience: 01 to 03 years in HPLC- Method Validation Department: AR&D- Method Development (Males) Qualification: M.Sc/ M.

Pharma Pathway
FEBRUARY 28, 2023
Analytical method development and Characterization using HPLC, GC, LCMS, GCMS, NMR, DSC, TGA, Raman Raman Spectroscopy and XRD in drug substance and drug products. Analytical method validations in GMP environment.

European Pharmaceutical Review
MARCH 6, 2024
Additionally, the new laboratory offers Ariceum the ability to carry out on-site process, method, validation and formulation development as well as preliminary stability analysis of its radiolabeled compounds.

Pharma Pathway
JANUARY 2, 2023
Experience in Analytical Method Development and Characterisation using HPLC, GC, LCMS, GCMS, NMR, DSC, TGA, Raman Spectroscopy and XRD in Drug substance and drug products. Experience in Analytical method validations in GMP environment. Date: 7th Jan’ 2023. Time: 09:30 AM to 02:30 PM.

Pharma Pathway
DECEMBER 14, 2022
Department: AR&D- (Method Validation). Experience: 01 to 04 years in HPLC- Method Validation. Department: R&D- (Synthesis / Process/ CRO). Qualification: M.Sc. Experience: 01 to 04 years. Qualification: M.Sc/ M. Pharmacy (Analytical Chemistry). Department: Regulatory Affairs (API). Qualification: M.Sc/ M.

GMPSOP
SEPTEMBER 27, 2023
Cleaning validation, including analytical method validation, should be conducted for the new product. Validate analytical method for cleaning validation After selecting the worst-case product check if an analytical method is validated for that product. Additional documents included each month.

Pharma Pathway
SEPTEMBER 14, 2022
Skill Set: Method Validation/ Verification analysis hands HPLC/ GC/ LCMS/ ICPMS etc., Experience : 02 to 08 years with relevant Experience. Walk-In for Quality Control -Officer/ Executive at Eye Drop Plant. Role & Responsibilities: . Required for Sterile Area: . and preference will be given to those are handling Empower software.

European Pharmaceutical Review
NOVEMBER 21, 2024
With over 22 years of experience, she has worked as a QC microbiologist in cell therapy and non-sterile pharmaceutical manufacturing, specialising in environmental monitoring and method validation. The post Understanding endotoxin cartridge testing: frequently asked questions appeared first on European Pharmaceutical Review.

Pharma Pathway
DECEMBER 31, 2022
Involved in Analytical method Validation. Role & Responsibilities: Responsible for IPQC, DS, DP and Stability sample testing on routine basis. Preparation of STP/ SOP/ DRS for QC Documentation. Instruments and Equipment handling for routine use as well as calibration and qualification.

European Pharmaceutical Review
JANUARY 25, 2024
Leachable studies leachables can stem from different sources and be formulation dependent” “Because leachables can stem from different sources and be formulation dependent” sufficient data should be provided to identify and characterise the potential risks associated with the leachables from the CCS.

Pharma Pathway
JANUARY 3, 2023
Skill Set: Stability & Method validation / verification analysis hands on experience HPLC/ GC/ LCMS/ ICPMS etc., Skill Set: Microbiology Analytical Activities, Reviewer, QMS documentation and Environmental Monitoring in Sterile manufacturing facility. Microbiology / BioTech. 5-12 years of relevant experience.

Pharma Pathway
JANUARY 30, 2023
Knowledge and handling of HPLC and GC Instruments activities. Knowledge and handling of Wet analysis (LOD, SOR, WC and TLC, Ex.) activities Troubleshooting for all handling instruments.

Pharma Pathway
JANUARY 2, 2023
Experience in Analytical Method Development and Characterisation using HPLC, GC, LCMS, GCMS, NMR, DSC, TGA, Raman Spectroscopy and XRD in Drug substance and drug products. Experience in Analytical method validations in GMP environment. Date: 7th Jan’ 2023. Time: 09:30 AM to 02:30 PM.

pharmaphorum
DECEMBER 16, 2021
Gain insight the current state of Endotoxin testing, including alternative test methods and strategic approaches to method validation. Key Reasons to Attend: Discover case studies in establishing holistic Contamination Control Strategies and Real-Time Viable Air Particle Counting with leaders in the pharmaceutical industry.
GMPSOP
MAY 31, 2024
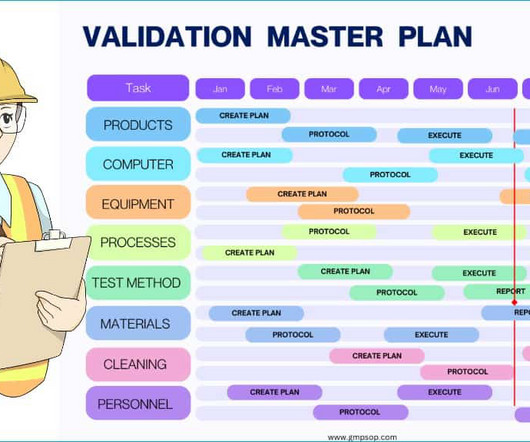
You can read our article “ Equipment Cleaning in Pharmaceutical ” to explore cleaning validation further. If you need help with complete guidance, please refer to the cleaning validation master plan. Method validation master plan? Create a master plan listing all products, specifications, and test methods.

Pharmaceutical Technology
AUGUST 24, 2022
To ensure the quality of FCM assay performance in preclinical and clinical applications, several scientific organizations developed recommendations for FCM instrumentation and method validation. The FDA and/or other regulatory agencies typically request validation reports to evaluate the assay performance for the intended purpose.2,3.

GMPSOP
OCTOBER 26, 2022
What is method validation in pharmaceutical industry? Analytical method validation involves demonstrating that the analytical methods used to test the quality of your product are accurate, reliable and reproducible which is critical for ensuring the quality and safety of your products.

Express Pharma
MAY 22, 2023
But, the excitement to leverage the growth potential in nutraceuticals has been countered by challenges like the lack of an effective approach/guideline for clinical proofs, clinical trials, factory audit standards, bioactive standardisation, method validations, etc.
GMPSOP
OCTOBER 28, 2023
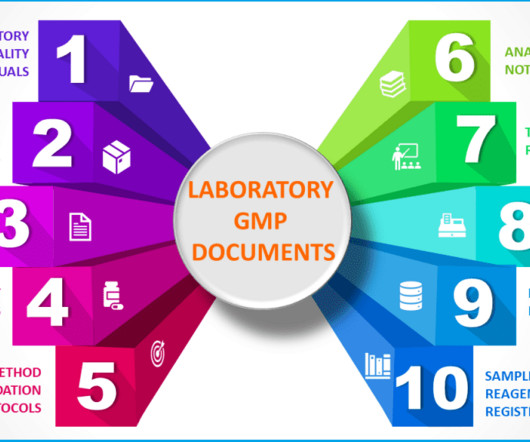
Validated test method A test method comprehensively describes all procedures used in sample analysis. Analytical test method validation guarantees that your test method is robust enough to provide evidence if your product meets the predetermined product specification or conforms to the failure of a product.

GMPSOP
APRIL 3, 2023
Adapted and non-standard methods must be validated. Protocols are only used for specific projects such as method validation testing or equipment qualification. – Methods for data analysis and interpretation, including the statistical techniques to be used.

epicur
JANUARY 18, 2024
While both BUDs and expiration dates serve a similar purpose of decreasing patient risks associated with any changes a medication may undergo during storage, expiration dates have key advantages : Expiration dates have a high degree of accuracy—they are determined using stability testing methods validated by cGMP regulations enforced by the FDA.
GMPSOP
NOVEMBER 19, 2023
All validation/qualification studies commenced or completed during the review period must be assessed. The effectiveness of the validation must be determined.
GMPSOP
JUNE 16, 2023
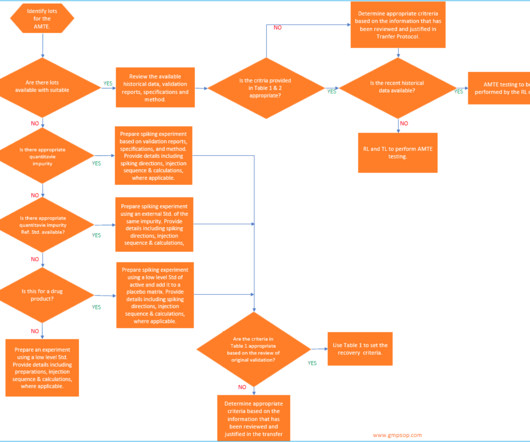
In this option, the RL has to assess all the risks and mitigate those risks by repeating the test parameters and matching the results against the original validation data. It’s essential to review the TL’s validation approach in accordance with current ICH Q2 guidelines and ensure that it covers the intended use of the method.

GMPSOP
JUNE 9, 2024
Test method validation in quality control Analytical Method Validation for Quality Control Test method validation generally means the same as analytical method validation, where the terms are interchangeable. Maintenance programs must be documented for laboratory equipment.

European Pharmaceutical Review
JANUARY 26, 2023
The European Medicines Agency (EMA) has published an updated Q&A document regarding ICH M10 ‘Bioanalytical Method Validation and Study Sample Analysis’. The ICH M10 guideline provides recommendations on the validation of bioanalytical assays for chemical and biological drugs and their metabolites in biological matrices.
GMPSOP
JULY 23, 2023
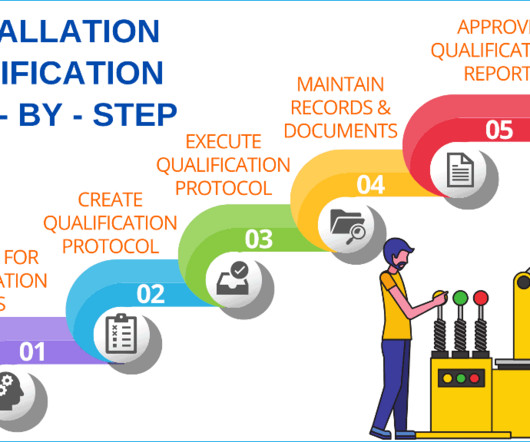
Validation of testing methods: Validate all testing methods used during the qualification process to ensure the accuracy and reliability of results. Well-documented processes: Maintain detailed records throughout the qualification process. 240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms.
GMPSOP
NOVEMBER 15, 2024
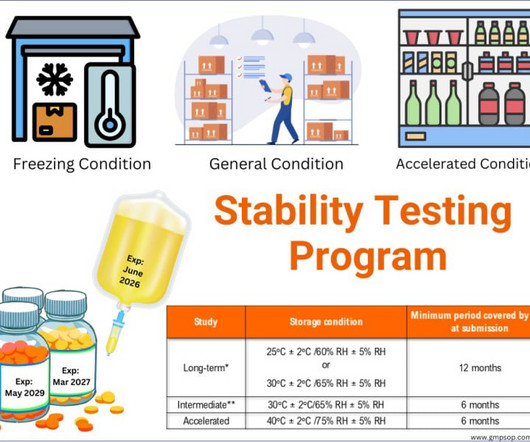
With the appropriate detector sensitivity, column, and mobile phase, stability-indicating methods are very useful for determining degradant concentrations. Forced degradation studies – method validation Forced degradation studies validate the analytical method as stability-indicating in nature.
GMPSOP
MARCH 4, 2023
If my cleaning methods are not validated would they cause contamination of my products? What is the probability my cleaning methods weren’t validated? Should I implement a more robust cleaning method validation to mitigate such risk? Do I have adequate control in place?

GMPSOP
DECEMBER 24, 2023
Laboratory documentation and records that should be available include: – Test methods and test reports – Laboratory notebooks/sheets, instrument records, and calculations – Conditions of tests and instrument settings. – Test method validation protocols, data and reports – Other records and data i.
Express Pharma
DECEMBER 23, 2024
The panel also discussed the role of analytical method validation when outsourcing testing and the necessity of Excel sheet validation. Chaudhary explained the necessity of sampling excipients and the exemption for dedicated facilities.

FDA Law Blog: Biosimilars
FEBRUARY 13, 2025
FDA recommends that the following information be made public regarding the PCCP as appropriate: planned modifications, testing methods, validation activities and performance requirements to meet before implementation, and means by which users will be informed of the implementation of the modification.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content