FDA rejects label expansion for Dynavax’s hepatitis B vaccine
Pharmaceutical Technology
MAY 15, 2024
The US agency rejected the expanded use of Dynavax’s vaccine for adults on haemodialysis, citing insufficient efficacy and safety data.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MAY 15, 2024
The US agency rejected the expanded use of Dynavax’s vaccine for adults on haemodialysis, citing insufficient efficacy and safety data.

Pharma Times
JULY 25, 2022
Imvanex to include protection from monkeypox and diseases caused by vaccinia virus
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
FEBRUARY 9, 2022
In this article, Ben Hargreaves looks into the promise of cancer vaccines and how this treatment modality may offer advantages over existing immunotherapies in the oncology sector. One area that is gathering increasing levels of interest is the development of cancer vaccines. A broad front.

pharmaphorum
MARCH 2, 2021
Zebra Technologies healthcare lead Chris Sullivan discusses the COVID-19 vaccine rollout and how technology can streamline the process to combat new variants. . While the rollout of the COVID-19 vaccine has signified the first signs of light at the end of the tunnel, the reality is that this public health crisis is still far from over.

Express Pharma
MAY 11, 2023
TechInvention Lifecare India, has partnered with Eubiologics Co, South Korea, to launch Euvichol-Plus, a oral cholera vaccine (OCV), in a low-density polyethylene (LDPE) unidose pack in India. The post TechInvention launches Euvichol-Plus, oral cholera vaccine in India appeared first on Express Pharma.

NY Times
APRIL 20, 2021
The European Union’s drug regulator said that the Johnson and Johnson vaccine should carry a warning of potential risk for rare blood clots on the label, but did not recommend stopping the vaccine, saying the benefits of the vaccine outweighed the risks.

pharmaphorum
OCTOBER 23, 2024
A new FDA approval has given Pfizer a broader label for its respiratory syncytial virus (RSV) vaccine Abrysvo than rival shots from GSK and Moderna, but it may not make much of a difference in the battle for market share.The US regulator has cleared Abrysvo for use in adults aged 18 to 59 at risk of RSV-related disease, extending its earlier label (..)

pharmaphorum
DECEMBER 16, 2022
Ben Hargreaves discovers why some have referred to the distribution of COVID-19 vaccines and treatments as a form of apartheid. Vaccine access for a price. Tendayi Achium, labelled the response by the global community as a form of “vaccine apartheid,” a suggestion echoed by the organisation’s director general.

Pharmaceutical Technology
OCTOBER 29, 2024
The registrational trial is expected to support the approval for Bavarian’s mpox/smallpox vaccine use in children 2-11 years of age.

Pharma in Brief
DECEMBER 3, 2020
Any authorization for drugs, vaccines, and medical devices issued pursuant to the interim orders are also set to terminate when the respective interim order expires or is withdrawn. Drugs and Vaccines. Both interim orders are set to expire 1 year after they were issued, or sooner if withdrawn by the Minister.

pharmaphorum
JULY 13, 2021
The FDA has added a new warning on the Johnson & Johnson COVID-19 vaccine, saying the shot has been linked to a serious but rare side effect – Guillain-Barré syndrome – that can cause muscle weakness. The cases have mostly been recorded two weeks after vaccination and seem to be more common in men aged 50 and older.

Pharmaceutical Technology
APRIL 6, 2023
Moderna has announced that its cancer vaccine mRNA-4157/V940 along with Keytruda secured the European Medicines Agency (EMA) Priority Medicines (PRIME) scheme designation for the adjuvant treatment of high-risk stage III/IV melanoma patients after complete resection.

pharmaphorum
JANUARY 28, 2021
The Covid-19 pandemic has put further pressure on clinical trial suppliers to be fleet of foot in getting products dispatched when a new patient is recruited, as studies for vaccines have been set up and run at unprecedented speed. . To find out more about the webinar and to register, visit [link] .

Pharmaceutical Technology
OCTOBER 23, 2024
Abrysvo is now approved to prevent lower respiratory tract disease caused by RSV in high-risk adults over 18 years of age.

FDA Law Blog: Biosimilars
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. A recent state law failure-to-warn case in the SDNY makes that very point.

Quality Matters
FEBRUARY 9, 2023
Africa’s growing vaccine capacity Manufacturing products locally for more equitable pandemic responses Every year, vaccines prevent nearly 5 million deaths around the globe. Yet, Africa produces less than 1 percent of the vaccines it needs to protect patients and communities across the continent.

pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford-AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.

pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford/AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.

pharmaphorum
MARCH 18, 2022
Moderna has asked the FDA for emergency use authorisation for a fourth dose of its mRNA COVID-19 vaccine SpikeVax, following in the footsteps of Pfizer/BioNTech, which filed their Comirnaty shot earlier this week. There’s no guidance yet from the CDC or FDA on whether a second booster will be needed.

Express Pharma
MAY 15, 2023
Ultragenyx has terminated its Phase I/II trial for pipeline candidate UX053, a non-vaccine mRNA therapeutic for glycogen storage disease type III, also known as Cori’s disease. According to GlobalData, UX053 was one of five non-vaccine mRNA therapeutics in clinical trial development.

PharmExec
JANUARY 9, 2025
Abrysvo and Arexvy will now be required to come with labeling that includes a warning about a potential increased risk of Guillain-Barr Syndrome.

Pharmaceutical Technology
OCTOBER 26, 2023
GSK plans to submit the data to regulatory agencies to support label expansion for Arexvy in the younger adult patient population in 2024.

Pharmaceutical Technology
JULY 29, 2022
The open-label, three-cohort, multicentre trial analysed intravesical BCG, N-803 combination in BCG-unresponsive high-grade NMIBC patients. In January, ImmunityBio and Amyris have concluded a joint venture (JV) agreement announced previously to accelerate the marketing of a Covid-19 vaccine.

Express Pharma
JANUARY 4, 2024
It meets 50 per cent of the global demand for various vaccines, 40 per cent of the generic demand in the US, and 25 per cent of all medicine in the UK. In addition to meeting strong domestic demand, the Indian pharmaceutical industry is well poised as a robust contributor to the growing global market.

pharmaphorum
APRIL 22, 2022
Astellas also revealed today that it is terminating the development of ASP2390, a DNA vaccine for house dust mite-induced allergic rhinitis, and GITR agonist antibody ASP1951 for cancer – both in early-stage clinical development – along with DMD gene therapies AT702, AT751 and AT753.

European Pharmaceutical Review
FEBRUARY 13, 2023
This is like antigens which can trigger an immune response and act as a vaccine. Firstly, using liquid chromatography and mass spectrometry, they carry out a systematic comparison of different fragments of a mRNA molecule to be tested with a similarly fragmented reference mRNA labelled with a stable carbon isotope.

Express Pharma
MAY 6, 2024
Florent Bouguin, CTO of Optel Group, uncovered how ultra-low temperatures, crucial for cryopreservation, are revolutionising mRNA production for next-generation medical interventions, particularly vaccines and therapies.

Pharmacy Is Right For Me
OCTOBER 21, 2022
My evenings involve music production, radio show production, podcast production, reviewing new music sent by labels/music promoters, and preparing for gigs. When the monkeypox outbreak was happening here in NYC, I used my platform to be a source of information about vaccines and TPOXX treatments.

pharmaphorum
DECEMBER 11, 2020
Advisers to the FDA have voted in favour of approving Pfizer and BioNTech’s COVID-19 vaccine, with a near-unanimous backing from an expert committee. Already approved in the UK, this is the first time that mRNA technology has been used to create a vaccine. Efficacy was consistent across age, gender, race and ethnicity demographics.

FDA Law Blog: Biosimilars
DECEMBER 3, 2023
The manufacturer’s agreement must cover all its labeler codes that contain an applicable drug or a selected drug. All labeler codes that are covered by Discount Program agreements will be distributed to PDP sponsors and posted on the CMS website. covered insulin product or vaccine). state pharmaceutical assistance programs).

Pharma Packaging Solutions
NOVEMBER 8, 2023
Pre-filled Syringe Packaging A lot of biopharmaceutical products—such as insulin, vaccines, and many hormone therapies—are parenterally administered. Vial and Ampoule Labeling Many biologics are available primarily in liquid form. Tjoapack handles crucial secondary packaging jobs like vial and ampoule labeling.
European Pharmaceutical Review
SEPTEMBER 22, 2023
Active anti-Abeta immunotherapies In the 2000s, the concept of active immunotherapy targeting Abeta in Alzheimer’s patients was assessed with the ELAN vaccine (AN1792), consisting of full-length aggregated Abeta 1-42 peptide mixed with the QS21 adjuvant. 8 However, as this trial was not placebo-controlled, conclusions are difficult to draw.

pharmaphorum
MARCH 16, 2022
Earlier this week, Pfizer chief executive Albert Bourla said that a second booster of its COVID-19 vaccine will be necessary to keep the pandemic under control, and the company has now asked the FDA to back this use. The real-world data comes from an analysis of health records from 1.1
Pharmaceutical Technology
SEPTEMBER 8, 2022
The list includes providers of development services, biologics management supply chain solutions, clinical trial services, commercial and logistics services, as well as packaging, labelling and distributing services for biologics.
Pharma Packaging Solutions
JUNE 30, 2023
Vaccines: Vaccines, many of which are grown in tissue cultures, use microorganisms to provide immunity for specific diseases, and are one of the most wide-spread types of biopharma products. We offer a variety of packaging services, including vial and ampule labeling, pharmaceutical kit assembly, and more.

Express Pharma
JULY 8, 2024
.” Prime Minister Narendra Modi has launched several groundbreaking programs in healthcare, such as Pradhan Mantri Jan Arogya Yojana, Pradhan Mantri Bhartiya Janaushadhi Pariyojana, and the successful COVID-19 vaccination drive.

Pharmaceutical Technology
JULY 14, 2022
Covid-19 vaccines stay in the spotlight. Even as regulatory agencies contemplate the authorization of Omicron-specific Covid-19 vaccines , existing versions continue to make headlines. Several companies have been charged with manufacturing different components of J&J’s vaccine.

GMPSOP
APRIL 3, 2024
A pharmaceutical warehouse is responsible for receiving, storing, and releasing incoming goods (including labeling and packaging) and distributing finished products. Materials and products may not need physical status labels or be stored in separate areas if computers are used. All these goods will be issued with unique lot numbers.

pharmaphorum
MAY 17, 2021
Apellis Pharma has secured FDA approval for its complement C3 inhibitor Empaveli as a treatment for paroxysmal nocturnal hemoglobinuria (PNH) – with a label that will allow it to challenge Alexion’s established therapies directly. .

Express Pharma
APRIL 13, 2023
In a world where ‘natural’ and ‘organic’ have become synonymous with sustainability, labelling a product ‘synthetic’ seems antithetical. As seen in the new mRNA-based COVID-19 vaccine delivery systems, synthetic lipid nanoparticles are providing stability throughout the delivery process and helping generate a stronger immunogenic response.

Pharma in Brief
JANUARY 19, 2023
We previously reported on the IO here.

FDA Law Blog: Biosimilars
JUNE 2, 2023
“All In” Manufacturer Definition CMS believes that the National Rebate Agreement (NRA) requires a “manufacturer” to report to Medicaid all of its covered outpatient drugs, under all of its labeler codes. This includes newly acquired labeler codes and newly formed subsidiaries.

pharmaphorum
FEBRUARY 26, 2021
Robert Wood’s granddaughter, Mary Lea, was the first baby to be used on the baby powder label. J&J’s size meant that it had the resources to bring to bear against COVID-19 as the pandemic ravaged the world in 2020 and 2021, and the company soon began work on its own vaccine in partnership with the US government.
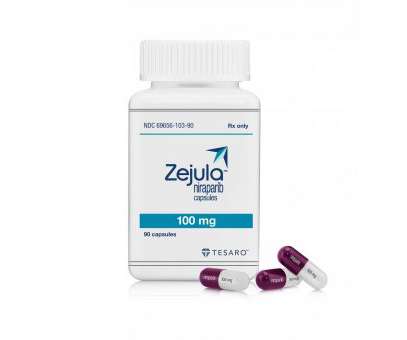
pharmaphorum
NOVEMBER 14, 2022
The narrower label for Zejula (niraparib) means it can only be used as second-line maintenance therapy after platinum-based chemotherapy in patients with these cancers whose tumours carry a germline BRCA mutation – around 15% of the population. That was due last Friday, but cancelled when GSK opted to withdraw the approval.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content