New Merck pneumococcal vaccine wins FDA approval
STAT
JUNE 17, 2024
The Food and Drug Administration on Monday approved Merck’s new pneumococcal vaccine for adults 18 and older.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

STAT
JUNE 17, 2024
The Food and Drug Administration on Monday approved Merck’s new pneumococcal vaccine for adults 18 and older.

STAT
SEPTEMBER 4, 2024
The standard advice — get your shots, get them at the same time, don’t wait too long to roll up your sleeves — is designed to maximize convenience and compliance, in a way that is manageable for the delivery enterprise, say experts familiar with the enormous effort it takes to turn vaccines into vaccinations.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
APRIL 18, 2024
A new startup focused on creating vaccines for cancers, influenza, and potentially even HIV launched Thursday with $54 million in funding. Altschuler and Ziff’s relationship goes back to the mid-2010s, when Altschuler was chief executive of the well-known research hospital Children’s Hospital of Philadelphia, or CHOP.
STAT
JANUARY 9, 2023
Moderna disclosed Monday that it plans to price its Covid-19 vaccine at anywhere from $110 to $130 per dose when the company pivots from a focus on government contracts to commercial distribution efforts. The timing was not disclosed, but the company is holding talks with hospitals, pharmacy chains and pharmacy benefit managers.

Pharma Times
DECEMBER 1, 2022
Novel tuberculosis vaccine tested in a large phase 3 trial against severe respiratory infection disease

STAT
MAY 4, 2023
Food and Drug Administration licensed the first-ever vaccine for respiratory syncytial virus, or RSV , completing an elusive quest that has been decades in the making , STAT writes. A vaccine that was developed by Pfizer and aimed at the same demographic is expected to be approved by the end of the month.

Pharma Times
DECEMBER 11, 2023
New data highlights the inequalities in emergency hospital rates and vaccinations - News - PharmaTimes

NY Times
AUGUST 3, 2021
The Food and Drug Administration’s move is expected to kick off more vaccination mandates for hospital workers, college students and federal troops.

NY Times
SEPTEMBER 17, 2021
The study found that the shots’ effectiveness in preventing hospitalization fell to 77 percent from 91 percent four months after full vaccination.

NY Times
AUGUST 23, 2021
The move was expected to kick off a round of new vaccination mandates from hospitals, schools and private companies.
European Pharmaceutical Review
OCTOBER 24, 2023
PENBRAYA (meningococcal groups A, B, C, W and Y vaccine), the first and only pentavalent vaccine that provides the broadest serogroup coverage of any meningococcal vaccine available in the US for meningococcal disease in individuals aged 10 to 25 years old, has been approved by the US Food and Drug Administration (FDA).

pharmaphorum
MARCH 2, 2021
Zebra Technologies healthcare lead Chris Sullivan discusses the COVID-19 vaccine rollout and how technology can streamline the process to combat new variants. . While the rollout of the COVID-19 vaccine has signified the first signs of light at the end of the tunnel, the reality is that this public health crisis is still far from over.

Express Pharma
SEPTEMBER 12, 2024
Indian Immunologicals (IIL), a vaccine manufacturer, has signed a Memorandum of Agreement (MoA) with the Indian Council of Medical Research (ICMR) to develop India’s first codon de-optimized live attenuated Zika vaccine. Currently, no vaccine is available for Zika prevention.

Express Pharma
SEPTEMBER 26, 2023
Indian Institute of Technology Mandi researchers, led by Dr Amit Prasad, Associate Professor, School of Biosciences and Bioengineering, has made strides in the development of vaccines against the pork tapeworm (T. Traditionally, tapeworm vaccines have been developed using products or antigens derived from tapeworm eggs or larvae.

pharmaphorum
DECEMBER 17, 2020
Trials of a fifth COVID-19 vaccine have begun in the UK, as IT issues threaten to delay the roll-out of the shot from Pfizer/BioNTech. France’s Valneva begun the phase 1/2 clinical study of the inactivated vaccine candidate VLA2001 in sites across the UK, supported by the National Institute for Health Research (NIHR).

The Pharmacist
MAY 4, 2023
A ground-breaking trial of a new Zika vaccine has started, with the first participant receiving the dose at the Royal Liverpool University Hospital, in conjunction with ongoing research at the universities of Liverpool and Manchester.

pharmaphorum
DECEMBER 9, 2020
Reviewers from the FDA have given their blessing to the Pfizer/BioNTech COVID-19 vaccine ahead of a key meeting tomorrow – but the regulator noted that there are still uncertainties about whether the shot can stop the disease from spreading. The post FDA reviewers back Pfizer/BioNTech COVID-19 vaccine ahead of panel appeared first on.

Community Pharmacy
OCTOBER 27, 2023
While COVID-19 might not be as pressing of a problem as it was during the peak of the pandemic, the virus and its many variants still pose significant health risks that require updated vaccine protection to combat. rise, which previous year’s vaccines were not designed to protect against.

Express Pharma
JUNE 15, 2023
The current influenza vaccines only provide protection against a limited number of strains, with the strains predicted to be dominant in the upcoming season selected for inclusion in the vaccines. Ultimately one of the biggest benefits of a universal influenza vaccine is that it could help to contain a potential pandemic.”

pharmaphorum
SEPTEMBER 7, 2020
A trial of Russia’s Sputnik V coronavirus vaccine has shown the jab produces an immune response, although the study was too small to produce conclusive findings, particularly on safety. The vaccine also produced a T-cell response within 28 days, a secondary outcome.

pharmaphorum
OCTOBER 23, 2020
Is a century-old vaccine a ‘game-changer’ for COVID-19? This is especially true in the search for effective therapies to fight COVID-19, and a vaccine. In a worst-case scenario, misplaced hype could lead to a sudden rush to buy doses of the BCG vaccine. Understanding the link between COVID-19 and the BCG vaccine.

pharmaphorum
JANUARY 31, 2022
It is becoming increasingly important for people all over the world to understand the importance of vaccines and to have access to the vaccines they need – especially with digital innovation driving new vaccine development. Pfizer’s Josh Raysman, Saad Saeed, and Shanaya Deboo discuss vaccine awareness, access, and innovation.
pharmaphorum
SEPTEMBER 24, 2020
The UK could be the first country to carry out a COVID-19 “challenge trial” where healthy volunteers are deliberately infected with coronavirus to test vaccines, according to press reports. That vaccine is top of the World Health Organization’s list of vaccines in clinical development, which shows there are 38 potential shots in human trials.

pharmaphorum
JUNE 2, 2021
In the latest edition of What HCPs Think , CREATION.co’s Laura Marsh looks at what healthcare professionals are saying about blood clots linked to COVID-19 vaccines. This sudden increase is due to reports of rare blood clots in recipients of the COVID-19 vaccines developed by AstraZeneca and more recently Johnson & Johnson.

pharmaphorum
JANUARY 27, 2021
A plant in Wales manufacturing the AstraZeneca COVID-19 vaccine was evacuated on the advice of the authorities today after it was sent a suspicious package. The contract covers production of 100 million doses of the AZ vaccine. Wockhardt UK in Wrexham this morning received a suspicious package.

Express Pharma
JULY 26, 2023
Results are based on analysis of data from 278,375 adults in New Zealand who got the vaccine between April 2018 and July 2021. Most were aged 70 or older Getting vaccinated against shingles could significantly reduce the risk of suffering a stroke or heart attack from the virus, a new study has found. Most were aged 70 or older.
pharmaphorum
DECEMBER 8, 2020
A 90-year-old woman has become the first person given a COVID-19 vaccine as part of the UK’s mass vaccination programme. Margaret Keenan was given the injection at 06:31 this morning at University Hospitals Coventry and Warwickshire NHS Trust. The post First COVID-19 vaccinations begin in UK hospitals appeared first on.

pharmaphorum
JUNE 27, 2021
AstraZeneca and Oxford University have started dosing patients in a trial of a new version of their COVID-19 vaccine that is designed to target the beta or South African variant of the SARS-CoV-2 virus. The post AZ starts phase 2/3 trial of new-variant COVID-19 vaccine appeared first on. The beta variant – also known as B.1.351
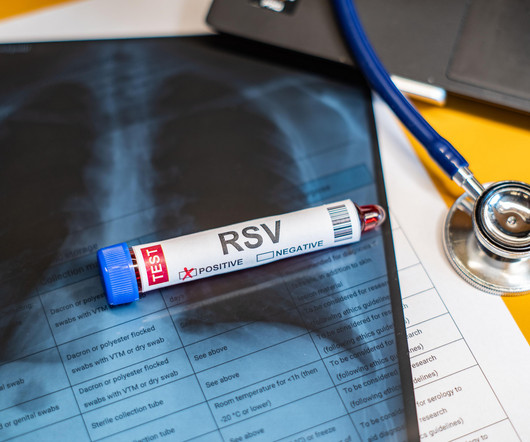
Pharmacy Times
SEPTEMBER 6, 2024
RSV vaccination was 75% effective in preventing RSV-associated hospitalizations among adults aged 60 years and older.

Pharmacy Times
NOVEMBER 13, 2024
RSV vaccination is approximately 77% to 81% effective against hospital and emergency department encounters among older adults.

Pharmacy Times
NOVEMBER 14, 2024
Increasing vaccine coverage among adults at highest risk could lessen associated hospitalizations and severe outcomes caused by RSV.

pharmaphorum
DECEMBER 30, 2020
The UK has approved AstraZeneca and Oxford University’s COVID-19 vaccine AZD1222 in another significant step forward in the fight against the pandemic, with first doses due to be administered on Monday. The #coronavirus vaccine is our way out of the pandemic – now we need to hold our nerve while we get through this together.
Pharmacy Times
DECEMBER 28, 2024
Study results show that the updated BNT162b2 XBB vaccine is effective in preventing hospitalizations or emergency department visits associated with COVID-19 complications.

pharmaphorum
MARCH 11, 2022
After a period where vaccine development had fallen away, suddenly it is back in the limelight. However, for those individuals working on vaccine development, the pandemic has proven to be a validation of the important work being carried out in the field. billion from its vaccine in 2021. The ‘poor relation’.
Pharmacy Times
NOVEMBER 8, 2024
Though both vaccines remain effective at preventing severe illness, Moderna’s mRNA-1273.222 vaccine was found to be significantly more effective among adults with underlying medical conditions.

Express Pharma
JUNE 20, 2023
The vaccine efforts of the COVID-19 pandemic highlighted our knowledge about gaps in the immune system. Similarly, institutes that facilitate connections between local hospitals and research institutes play an essential role. However, the spotlight has shifted to unravelling the complexities of the human immune system.

Big Molecule Watch
SEPTEMBER 3, 2024
On August 22, Moderna announced that FDA approved its supplemental Biologics License Application (“sBLA”) for a new formula of their COVID-19 vaccine, SPIKEVAX®, for individuals 12 years old and above. Emergency Use Authorization (“EUA”) was also granted for the updated vaccine for individuals aged 6 months to 11 years old.

Pharmacy Times
MARCH 3, 2025
New results shed light on the ineffectiveness of the combination antiviral therapy in patients vaccinated against COVID-19.

PharmaTech
APRIL 6, 2023
Nicole Lewis, PVP Program Manager at Boston Children's Hospital, discusses the science behind precision vaccines, a form of personalized vaccines.

Eye on FDA
NOVEMBER 17, 2020
Coincident to COVID-19 case levels in Europe and the United States entering new and serious levels comes welcome news on the development of a vaccine. While vaccination may actually begin in December, the number of doses will be limited. In addition, the process of vaccination takes time.
Pharmacy Times
AUGUST 30, 2022
Hospitalization and interprofessional team management, particularly the involvement of a pharmacist, are essential in vaccination coverage for patients with diabetes.

Express Pharma
JANUARY 20, 2024
Indian Immunologicals has launched Hepatitis A vaccine “Havisure”. It is a two-dose vaccine wherein the first dose is administered at above 12 months of age and the second dose is given at least after 6 months of the first dose. The vaccine is comparable to the world’s leading vaccine sold by a multinational.

Pharmacy Times
OCTOBER 5, 2022
Vaccines reduce the risk of COVID-19 infection and hospitalization, but a recent study suggests that misinformation could make adults hesitant to vaccinate children 5 to 11 years of age.
Pharmaceutical Technology
DECEMBER 9, 2022
The European Commission (EC) has granted marketing authorisation for Takeda ’s Qdenga (Dengue Tetravalent Vaccine [Live, Attenuated]) (TAK-003) to prevent dengue in people aged four years and above. According to the results, the trial met the primary endpoint of overall vaccine efficacy by averting 80.2%
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content