Johnson & Johnson Will Test its Vaccine in Infants
NY Times
FEBRUARY 28, 2021
The company’s coming trials will also involve pregnant women and people whose immune systems are compromised.

NY Times
FEBRUARY 28, 2021
The company’s coming trials will also involve pregnant women and people whose immune systems are compromised.

Outsourcing Pharma
MARCH 3, 2021
The 2020 Pfizer/BioNTech COVID-19 vaccine trial recruited more than 44,000 participants and reached submission for emergency authorization in 248 days: showing unprecedented speed and agility in the context of a global pandemic. So what have been the learnings from the trial â and how could they be applied to vaccine development in the future?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharma Times
MARCH 2, 2021
Charge will increase to £9.

pharmaphorum
MARCH 3, 2021
Rare disease families need to stand up and be counted if they want things to change said mum and advocate Christine Waggoner. She spoke to pharmaphorum as part of our Patients Insights series. Like many “rare disease mums”, Christine is an accidental advocate. After her daughter, Iris, was diagnosed with a rare disease at five, friends, family, and strangers donated so much to a research fund-raising campaign that she had to set up a non-profit to deal with it all.

Speaker: Jennifer Hill
Payroll compliance is a cornerstone of business success, yet for small and midsize businesses, it’s becoming increasingly challenging to navigate the ever-evolving landscape of federal, state, and local regulations. Mistakes can lead to costly penalties and operational disruptions, making it essential to adopt advanced solutions that ensure accuracy and efficiency.

NY Times
MARCH 4, 2021
Johnson & Johnson’s one-shot vaccine is allowing states to rethink distribution, even as health officials and experts worry some will view it as inferior.
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Pharma Times
MARCH 5, 2021
UK’s regulatory authority will fast-track jabs modified to new variants

pharmaphorum
MARCH 2, 2021
Zebra Technologies healthcare lead Chris Sullivan discusses the COVID-19 vaccine rollout and how technology can streamline the process to combat new variants. . While the rollout of the COVID-19 vaccine has signified the first signs of light at the end of the tunnel, the reality is that this public health crisis is still far from over. More than 100 million people worldwide have been vaccinated thus far, but several new variants of the virus have emerged in South Africa, Brazil and the United Ki

NY Times
MARCH 4, 2021
Interim results from clinical trials suggested that Covaxin could be safe and effective, potentially removing one hurdle to New Delhi’s ambitious campaign to inoculate a vast population.
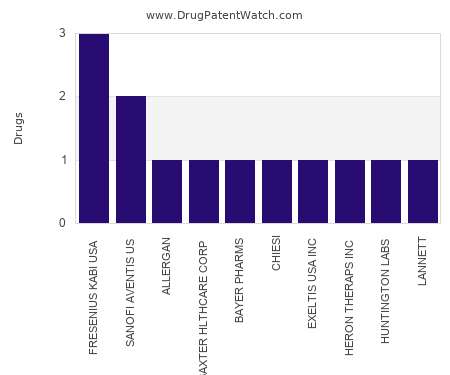
Drug Patent Watch
MARCH 5, 2021
This chart shows the pharmaceutical companies with the most emulsion dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most emulsion dosed drugs…. The post Which pharmaceutical companies have the most emulsion dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharma Times
MARCH 4, 2021
Rolling review begins based on lab and clinical studies in adults

pharmaphorum
MARCH 2, 2021
Clinical trial software facilitates clinical trials from conception to finish. For example protocol management, CRF design , metadata management, and the collection, analysis, and submission of compliant clinical study data to regulatory authorities. The aim is to get quality clinical products to the market faster. Traditionally spreadsheets have been used to record and manage all the various aspects of clinical trials.

Outsourcing Pharma
MARCH 1, 2021
A leader from the genetic solutions firm explains how genetic testing and counseling can help trial teams form more beneficial connections with patients.

Pharma Marketing Network
MARCH 1, 2021
[link]. Blockchain technology has become an increasing tool for life science organizations. How is this technology being implemented, and what value does it offer? Join Darshan Kulkarni as he talks with guest Jim Nasr about the function of blockchain, and how distributed ledger technologies such as blockchain are making an impact on healthcare. The post DarshanTalks: Blockchain & Information Technology</br> – March 1, 2021 appeared first on Pharma Marketing Network.

Advertisement
Managing HR tasks like payroll, compliance, and employee data can overwhelm small businesses. That’s where a Human Capital Management (HCM) solution comes in. Our eBook, Why Every Small Business Needs an HCM Solution: A Comprehensive Guide , shows how an HCM system automates tedious processes, ensuring your business stays compliant and efficient. You’ll learn how to simplify payroll, eliminate costly errors, and empower your employees with self-service tools.

Pharma Times
MARCH 1, 2021
Latest funding round includes investments from Foresite Capital and F-Prime Capital

pharmaphorum
MARCH 4, 2021
AI can be transformative for all areas of healthcare, but often these systems are built off biased datasets that don’t reflect the true diversity of the general public – leading to approaches that only work for specific populations. Intouch Group’s Abid Rahman explains this concept of AI equity and gives some practical tips for how companies can work towards it.

Outsourcing Pharma
MARCH 4, 2021
The CRO will receive funding to fuel a five-year Research Project Award, with the goal to design and conduct research of potential PTSD treatment options.

Drug Patent Watch
MARCH 4, 2021
Annual Drug Patent Expirations for PROCYSBI Procysbi is a drug marketed by Horizon Pharma Usa and is included in two NDAs. There are sixteen patents protecting this drug. Drug patent…. The post New patent for Horizon Pharma drug PROCYSBI appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharma Times
MARCH 4, 2021
Collaboration will focus on vaccines for infectious diseases

pharmaphorum
MARCH 2, 2021
UK chancellor Rishi Sunak has been urged to recognise the leading role that pharma companies are playing in the economy in this week’s budget. Sunak is due to make his closely-watched budget speech tomorrow (Wednesday) in the House of Commons, starting from about 12.30. The Conservative Party has strong ties with pharma and life sciences and has been backing the industry since David Cameron led it back to power in 2010 with a range of policies aimed at boosting research and investment.

Pharma Marketing Network
MARCH 3, 2021
We’ve talked before about how COVID-19 has upended our personal and business lives, and how the pharma industry was able to – impressively – shift gears to meet the challenges the pandemic brought. But what about patients? And what about the disrupted patient journey? What can our industry do to ensure patients maintain continuity of care at a time when good health is imperative?

Pharma in Brief
MARCH 1, 2021
On February 24, 2021, Parliament voted down Bill C-213 , which would have enacted the Canada Pharmacare Act. The Private Member’s Bill was first introduced a year ago, re-introduced in September 2020 after Parliament’s prorogation, and defeated at Second Reading. The Canada Pharmacare Act set out a series of criteria and conditions that would have been required before a federal cash contribution could be provided to a province for its public drug insurance plan: public administration; comprehens

Speaker: Joe Sharpe and James Carlson
Payroll optimization can be one of the most time-consuming and complex factors of small business management. Yet, organizations that crack the code on streamlining employee compensation often discover innovative avenues for growth. With the right strategies in place, outsourcing and streamlining payroll processes can result in substantial time and resource savings.

Pharma Times
MARCH 1, 2021
First therapy approved for the ultra-rare and progressive condition

pharmaphorum
MARCH 3, 2021
ISA Pharmaceuticals is planning to take its COVID-19 immunotherapy into the clinic, aiming to harness the power of T-cells to prevent the disease from becoming serious in infected patients. Based in Leiden, the Netherlands, ISA said it has completed preclinical work on ISA106 and is planning clinical trials with a phase 1 dose-finding study in healthy volunteers due in the next few months.

Outsourcing Pharma
MARCH 2, 2021
The Verisense Pulse+ can measure heart rate, oxygen saturation, emotional responses, and activity and sleep levels of at-home clinical trial participants.

NY Times
MARCH 1, 2021
Much is still to be determined about how this new tool will be used, but some things are already clear.

Advertisement
Running a healthcare facility requires precision and care, not just for patients but also for your staff. Our guide, "A Buyer’s Guide to Payroll & HCM Services," helps healthcare providers choose the best provider. Efficient payroll management ensures timely, accurate payments, critical for maintaining staff morale and trust. Compliance support helps navigate complex healthcare regulations and avoid costly fines.
Let's personalize your content