New coalition formed to raise awareness of sight loss
Pharma Times
APRIL 20, 2022
‘The Eyes Have It’ partnership will highlight the importance of eye health

Pharma Times
APRIL 20, 2022
‘The Eyes Have It’ partnership will highlight the importance of eye health

pharmaphorum
APRIL 21, 2022
A study has found that there is still a very high level of inappropriate antibiotic prescribing to patients in the US, particularly to older and Black patients. Sifting through data from around seven billion outpatient visits to doctor’s offices, hospital clinics and emergency departments over a seven-year period, the researchers found that almost three-quarters (74%) of antibiotics prescribed to patients aged 65 years or older, and two thirds (64%) to Black patients, were unnecessary.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
APRIL 20, 2022
The two-day Oracle Health Sciences Connect will gather an array of research experts to discuss how to make the most of emerging technologies and practices.
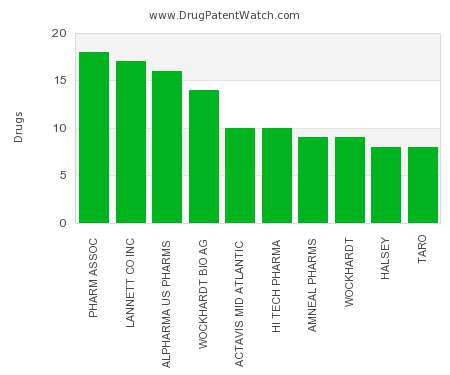
Drug Patent Watch
APRIL 22, 2022
This chart shows the pharmaceutical companies with the most syrup dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most syrup dosed drugs…. The post Which pharmaceutical companies have the most syrup dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Jennifer Hill
Payroll compliance is a cornerstone of business success, yet for small and midsize businesses, it’s becoming increasingly challenging to navigate the ever-evolving landscape of federal, state, and local regulations. Mistakes can lead to costly penalties and operational disruptions, making it essential to adopt advanced solutions that ensure accuracy and efficiency.

Pharma Times
APRIL 22, 2022
Researchers were able to detect specific combinations of genetic alterations which may hold key to the growth of cancers
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Outsourcing Pharma
APRIL 20, 2022
An expert from the health tech company explains how AI, natural language processing, and other tools can improve pharmaceutical product safety reporting.

Drug Channels
APRIL 20, 2022
This week, I’m rerunning some popular posts while I prepare for this Friday’s live video webinar: PBM Industry Update: Trends, Controversies, and Outlook. Click here to see the original post and comments from January 2022. For 2022, the three largest pharmacy benefit managers (PBMs)—Caremark (CVS Health), Express Scripts (Cigna), and OptumRx (United Health Group)— increased the number of drugs they excluded from their standard formularies.

Pharma Times
APRIL 22, 2022
Infertility is estimated to affect one in seven couples across the UK and can have a profound effect on mental health

pharmaphorum
APRIL 22, 2022
Ampio Pharma’s candidate therapy for knee osteoarthritis has been knocked back by the FDA, which will likely now require a new clinical trial of the drug before it will consider a review. Shares in the US biotech have fallen sharply after it revealed that the regulator did not accept changes to a phase 3 trial of the drug – called Ampion – that the company put forward in order to make it serve as a confirmatory pivotal study.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

NY Times
APRIL 16, 2022
It needs to promote innovation, not reward legal trickery.

Drug Channels
APRIL 22, 2022
This week, I’m rerunning some popular posts while I prepare for today’s live video webinar: PBM Industry Update: Trends, Controversies, and Outlook. Click here to see the original post and comments from February 2022. More than four years ago, I warned about the emerging trend of copay accumulators and outlined the costly consequences for patients. The latest data reveal that copay accumulator adjustment programs are now in the word list for a growing share of pharmacy benefit designs.

Pharma Times
APRIL 19, 2022
Protein technology could dramatically improve prospects of heart attack victims by proliferating organ cells

pharmaphorum
APRIL 19, 2022
Ehab Youssef, area head of Europe North at Roche Pharmaceuticals, tells us why European countries need interoperability to ensure a more effective overall European healthcare system. COVID spotlighted the vulnerability of the healthcare system and the need for structural and technological change to help manage such as crisis. Though some regions have started to make the necessary changes, Youssef says Europe is lagging. “In general, there is a good progression in almost every country.

Advertisement
Managing HR tasks like payroll, compliance, and employee data can overwhelm small businesses. That’s where a Human Capital Management (HCM) solution comes in. Our eBook, Why Every Small Business Needs an HCM Solution: A Comprehensive Guide , shows how an HCM system automates tedious processes, ensuring your business stays compliant and efficient. You’ll learn how to simplify payroll, eliminate costly errors, and empower your employees with self-service tools.

NY Times
APRIL 22, 2022
Once again, a dysfunctional health care system has hindered our pandemic response.

Drug Channels
APRIL 21, 2022
This week, I’m rerunning some popular posts while I prepare for tomorrow’s live video webinar: PBM Industry Update: Trends, Controversies, and Outlook. Click here to see the original post and comments from October 2021. By reader request, we present below a channel flow chart illustrating the buy-and-bill process for provider-administered drugs. This chart complements Follow the Dollar: The U.S.

Pharma Times
APRIL 21, 2022
The new guidelines focus on key areas of prescription, management of withdrawal and severity of withdrawal symptoms

pharmaphorum
APRIL 22, 2022
Astellas has said it will book a $170 million impairment charge in its fourth quarter results as a result of a decision to halt the development of three gene therapy candidates for Duchenne muscular dystrophy in preclinical development. The write-down comes in the wake of problems affecting the Japanese drugmaker’s AT132 gene therapy candidate for rare disease X-linked myotubular myopathy (XLMTM), which was placed on clinical hold by the FDA last year after four patient deaths liked to pos

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Outsourcing Pharma
APRIL 19, 2022
Cryoport Inc has acquired Cell&Co BioServices, continuing its EMEA expansion efforts.

Drug Patent Watch
APRIL 18, 2022
This chart shows the companies which have received the most New Patient Population exclusivities in the past five years. New Patient Populations are one of the categories for which the…. The post Pharmaceutical companies with the most ‘New Patient Population’ drugs appeared first on DrugPatentWatch - Make Better Decisions.

Pharma Times
APRIL 19, 2022
High unmet need in the area of hepatitis B therapies will see companies share responsibilities to develop the vaccine

pharmaphorum
APRIL 19, 2022
AstraZeneca and Daiichi Sankyo should only have to wait for six months to hear from the FDA if it will approve their HER2 drug Enhertu for non-small cell lung cancer (NSCLC) and add to its current uses in breast and gastric cancer. The US regulator has kicked off a priority review of Enhertu (trastuzumab deruxtecan) as a treatment for NSCLC with HER2 mutations after prior systemic therapy, setting up a decision in the third quarter of this year.

Speaker: Joe Sharpe and James Carlson
Payroll optimization can be one of the most time-consuming and complex factors of small business management. Yet, organizations that crack the code on streamlining employee compensation often discover innovative avenues for growth. With the right strategies in place, outsourcing and streamlining payroll processes can result in substantial time and resource savings.

Outsourcing Pharma
APRIL 21, 2022
The latest news on expansions, innovations, awards, and more includes items from Eversana, DHL Supply Chain, X-Chem, Medidata, and other notable companies.

Drug Channels
APRIL 19, 2022
This week, I’m rerunning some popular posts while I prepare for this Friday’s live video webinar: PBM Industry Update: Trends, Controversies, and Outlook. Click here to see the original post and comments from November 2021. An ironic postcript: Less than two months after my article was published, Cigna CEO David Cordani bragged about the cost savings from "aggressive adoption" of biosimilars.

Pharma Times
APRIL 20, 2022
Company at the forefront of developing personalised treatments against invasive cancer has raised 15m euros to conduct clinical trials

pharmaphorum
APRIL 20, 2022
A meta-analysis of published studies looking at web- app- or telehealth-based therapy for osteoarthritis has concluded that they outperform standard approaches to care. The study – published in the journal Osteoarthritis and Cartilage – focused on patients with osteoarthritis of the hip or knee, and compared the digitally-delivered exercises to standard approaches such as in-person physiotherapy, other forms of care, waitlisting and patient education.

Advertisement
Running a healthcare facility requires precision and care, not just for patients but also for your staff. Our guide, "A Buyer’s Guide to Payroll & HCM Services," helps healthcare providers choose the best provider. Efficient payroll management ensures timely, accurate payments, critical for maintaining staff morale and trust. Compliance support helps navigate complex healthcare regulations and avoid costly fines.
Let's personalize your content