UK trial to evaluate existing drugs for secondary breast cancer
Pharma Times
SEPTEMBER 24, 2021
Trial will test if radium-223 plus avelumab can improve outcomes for metastatic breast cancer patients

Pharma Times
SEPTEMBER 24, 2021
Trial will test if radium-223 plus avelumab can improve outcomes for metastatic breast cancer patients

pharmaphorum
SEPTEMBER 23, 2021
A study has found that listening to a specially composed music track can achieve “clinically significant” reductions in pain intensity and unpleasantness, according to the researchers behind the work. The All of Us track – composed by musician Anatole with the help of psychologist Dr Claire Howlin of University College Dublin and available via Spotify – reportedly showed a benefit in 286 people suffering from various types of acute pain, including headache, backache or period pain in
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
SEPTEMBER 21, 2021
According to a leader from the health engagement solutions firm, intelligent use of technology tools can add patient-centricity and realism to a study.

Drug Patent Watch
SEPTEMBER 20, 2021
I’ll be leading a workshop on generic portfolio management, and also giving a talk, at the 14th annual Marcus Evans event on Portfolio Planning and Partnerships for Generics. The event…. The post Workshop and lecture on generic portfolio management appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Jennifer Hill
Payroll compliance is a cornerstone of business success, yet for small and midsize businesses, it’s becoming increasingly challenging to navigate the ever-evolving landscape of federal, state, and local regulations. Mistakes can lead to costly penalties and operational disruptions, making it essential to adopt advanced solutions that ensure accuracy and efficiency.

Pharma Times
SEPTEMBER 20, 2021
Drug combination could offer a new option for treatment-resistant advanced ovarian cancer
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

Pharma Mirror
SEPTEMBER 18, 2021
25th September is celebrated as world pharmacists day, and it brings a new theme every year. The main motive behind this celebration is to give an excellent message to improve people’s health worldwide. And also, create an acceptance and encourage the role of a pharmacist in health. The pharmacist community from all over the world arranges different campaigns, exhibitions, lectures, etc., for serving this purpose.

Outsourcing Pharma
SEPTEMBER 22, 2021
A logistics leader from the CRO offers advice on navigating borders, cultures, regulatory issues and other important considerations on worldwide studies.

Pharma Times
SEPTEMBER 21, 2021
Ronapreve will be targeted at hospitalised patients who have not mounted an antibody response against COVID-19

pharmaphorum
SEPTEMBER 24, 2021
An NHS study of a new cancer blood test holds huge promise, but will not be without its challenges, says Snedden Campbell’s Ivor Campbell. The announcement that the NHS is taking part in a major new clinical trial for a blood test that could detect more than 50 cancers early has been welcomed as a potential, once-in-a-generation breakthrough. The UK health service partner with Silicon Valley-based healthcare company Grail in a randomised control trial involving up to 140,000 volunteers.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Channels
SEPTEMBER 23, 2021
This week, I’m rerunning some popular posts while I prepare for this Friday’s live video webinar: Drug Channels Update: Buy-and-Bill Market Trends. Click here to see the original post and comments from May 2021. Last week, Cigna released its earnings for the first quarter of 2021. Links below. I was struck by how quickly Cigna’s Express Scripts PBM business has increased revenues and prescriptions from its retail pharmacy network.

NY Times
SEPTEMBER 18, 2021
Competition for market share at rock-bottom prices has led to shortages, price-spikes, allegations of price-fixing, and substandard and even dangerous practices.

Pharma Times
SEPTEMBER 20, 2021
'Groundbreaking results' for the treatment of HER2-positive metastatic breast cancer

pharmaphorum
SEPTEMBER 23, 2021
Novavax and its partner Serum Institute of India have applied to the World Health Organization for an emergency use listing for their COVID-19 vaccine, based on a conventional recombinant protein technology. Getting a WHO emergency use listing (EUL) is a requirement for approvals by many national regulatory authorities, as well as for participation in the COVAX mechanism, which has been set up to procure COVID-19 vaccines for lower-income countries.

Advertisement
Managing HR tasks like payroll, compliance, and employee data can overwhelm small businesses. That’s where a Human Capital Management (HCM) solution comes in. Our eBook, Why Every Small Business Needs an HCM Solution: A Comprehensive Guide , shows how an HCM system automates tedious processes, ensuring your business stays compliant and efficient. You’ll learn how to simplify payroll, eliminate costly errors, and empower your employees with self-service tools.

Drug Patent Watch
SEPTEMBER 24, 2021
Annual Drug Patent Expirations for GIAPREZA Giapreza is a drug marketed by La Jolla Pharma and is included in one NDA. It is available from one supplier. There are eight…. The post New patent for La Jolla drug GIAPREZA appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
SEPTEMBER 20, 2021
Informa Connect's Pharma/Biotech GTN Summit. Hybrid Event In-Person: November 17-19, 2021 in Philadelphia Virtual: November 22-23, 2021 www.informaconnect.com/gtn. Join the life sciences community this fall at Informa Connect’s GTN Summit , now in the 11th year. Whether you register with an All-Access Pass (attending in-person on November 17-19, 2021 with access to Virtual content) or register with a Virtual Pass (attending from your home or office on November 22-23, 2021 with access to the In-P

Pharma Times
SEPTEMBER 21, 2021
Cabometyx found to reduce the risk of disease progression or death versus placebo in this patient population

pharmaphorum
SEPTEMBER 21, 2021
RwHealth , the leading provider of Artificial Intelligence to the healthcare industry, has today reported 160 per cent year-on-year growth as its data-led solutions are increasingly embraced by NHS trusts, private healthcare providers and major pharmaceutical companies. RwHealth’s unique Data Science Platform combines Artificial Intelligence (AI), Machine Learning and Data Science to give healthcare providers access to the best and most in-depth data available, helping them to make better decisi

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
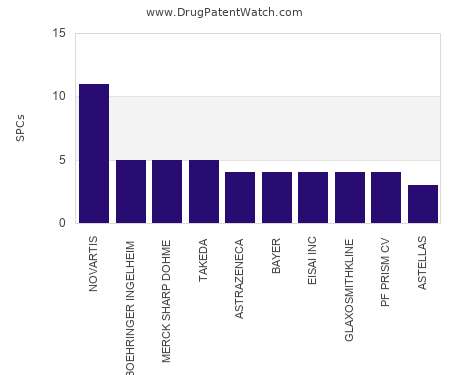
Drug Patent Watch
SEPTEMBER 18, 2021
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Finland. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Finland? appeared first on DrugPatentWatch - Make Better Decisions.

NY Times
SEPTEMBER 21, 2021
The pandemic has exposed flaws in services for people who can’t easily access a drive-through window for Covid shots or testing or can’t read prescription labels.

Pharma Times
SEPTEMBER 24, 2021
Results showed treatment with Crysvita reduces disease burden for patients

pharmaphorum
SEPTEMBER 22, 2021
The third generation of his family to hold a leadership role at Chiesi Group, Giacomo Chiesi reflects on the lessons he learned from his father and grandfather – and from his work as a former management consultant – about what it means to run a successful family business and its benefits. Family businesses across industries including the life sciences sector contribute significantly to the global economy – they represent about half of the world gross domestic product and global emplo

Speaker: Joe Sharpe and James Carlson
Payroll optimization can be one of the most time-consuming and complex factors of small business management. Yet, organizations that crack the code on streamlining employee compensation often discover innovative avenues for growth. With the right strategies in place, outsourcing and streamlining payroll processes can result in substantial time and resource savings.

Drug Patent Watch
SEPTEMBER 23, 2021
Annual Drug Patent Expirations for SAXENDA Saxenda is a drug marketed by Novo and is included in one NDA. It is available from two suppliers. There are twenty-two patents protecting…. The post New patent for NOVO drug SAXENDA appeared first on DrugPatentWatch - Make Better Decisions.

NY Times
SEPTEMBER 23, 2021
Scientific advisers to the C.D.C. have been discussing the details of federal guidance on who qualifies for a Pfizer-BioNTech booster shot and why. A decision is expected on Thursday.

Pharma Times
SEPTEMBER 21, 2021
Adagrasib is an investigational oral small-molecule inhibitor of KRAS G12C

pharmaphorum
SEPTEMBER 22, 2021
Software that can help pathologists detect prostate cancer from slides of biopsies more effectively has been approved by the FDA. The software – called Paige Prostate – is the first artificial intelligence-based to be approved by the FDA for this purpose, according to the US regulator. It is used to screen for signs of cancer on biopsy slides that have been digitised using a scanner, identifying those with the highest risk so they can be reviewed further by a pathologist if not spotted on an ini

Advertisement
Running a healthcare facility requires precision and care, not just for patients but also for your staff. Our guide, "A Buyer’s Guide to Payroll & HCM Services," helps healthcare providers choose the best provider. Efficient payroll management ensures timely, accurate payments, critical for maintaining staff morale and trust. Compliance support helps navigate complex healthcare regulations and avoid costly fines.
Let's personalize your content