Labetalol batch recalled due to wrong dosage labelling
The Pharmacist
JULY 10, 2023
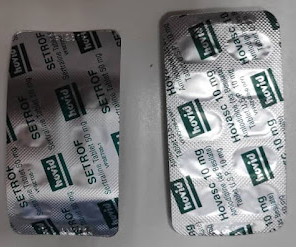
A batch of Labetalol 200mg tablets has been recalled as they are incorrectly labelled as 100mg tablets on the foil blister packaging. Pharmacists have been instructed to immediately stop […] The post Labetalol batch recalled due to wrong dosage labelling appeared first on The Pharmacist.









































Let's personalize your content