Common pharma compliance concerns cited by FDA warning letters
European Pharmaceutical Review
AUGUST 23, 2022
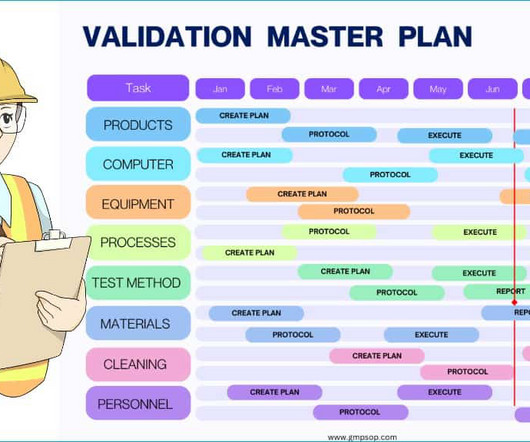
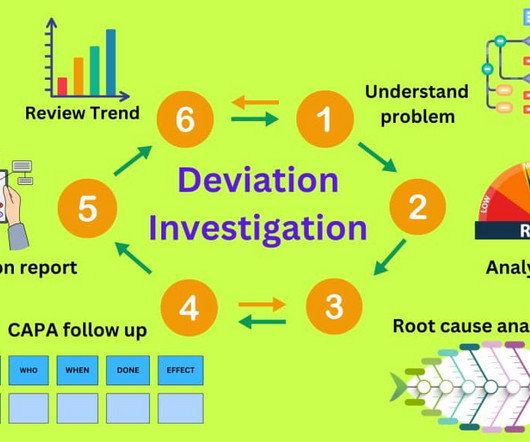
Of the cGMP letters, three major types of violations – deficiencies in process validation, documentation practices (data integrity) and quality control – accounted for 26 percent, 21 percent and 15 percent of warning letters respectively. Overall, validation, documentation and quality control were the major cGMP violations.




















Let's personalize your content