How to conduct product quality review in pharmaceutical
GMPSOP
NOVEMBER 19, 2023
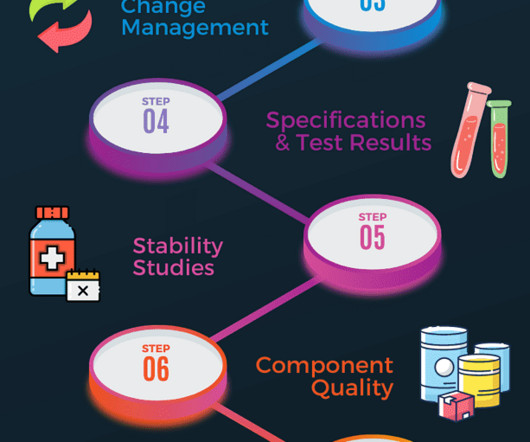
You should perform product quality review annually to determine if any adjustments to product specifications, manufacturing process or raw material supply are required to improve the product further. The content of this article does not supersede or replace any local or international regulatory requirements.







Let's personalize your content