Overview of pharmaceutical quality control steps and implementations
GMPSOP
JUNE 9, 2024
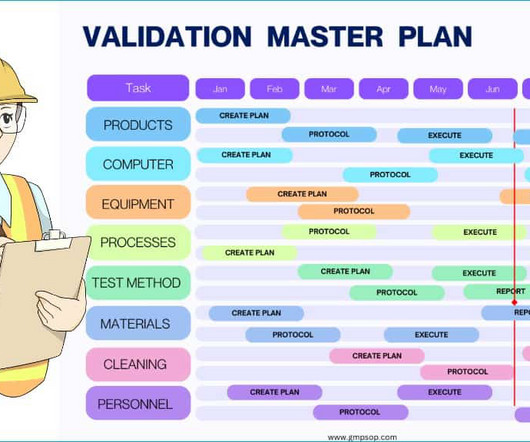
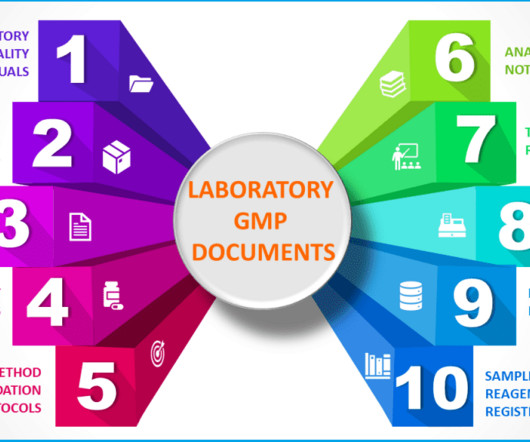
From starting materials to testing pharmaceutical products coming out of the manufacturing process, quality control is the only function that detects unknown defects early and allows for timely correction. This means that the analytical methods consistently generate true results with precision and accuracy every time.









Let's personalize your content