Overview of pharmaceutical quality control steps and implementations
GMPSOP
JUNE 9, 2024
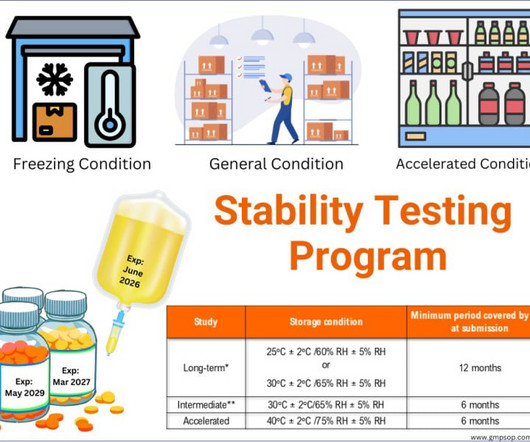
This article will focus on some of the common activities performed in quality control facilities daily to maintain the expected level of compliance and ensuring product quality. This means that the analytical methods consistently generate true results with precision and accuracy every time. accuracy, precision, etc.)









Let's personalize your content