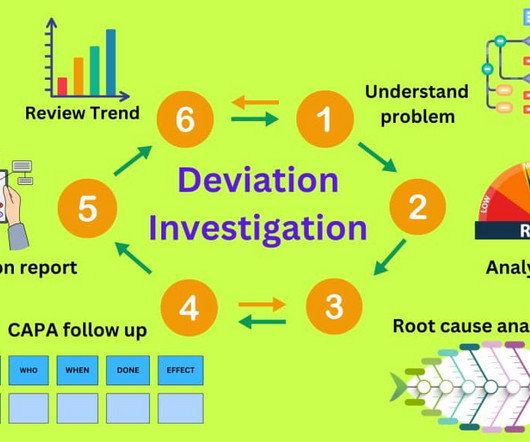
Deviation investigation guidelines in the pharmaceutical industry
GMPSOP
JUNE 28, 2024
This article will explore more elements of unplanned deviations than planned deviations. It is important to understand that everyone in the pharmaceutical manufacturing facility is responsible for reporting a deviation as soon as one is identified. incorrect data entry).







Let's personalize your content