What are the elements of quality control process in pharmaceuticals
GMPSOP
OCTOBER 28, 2023
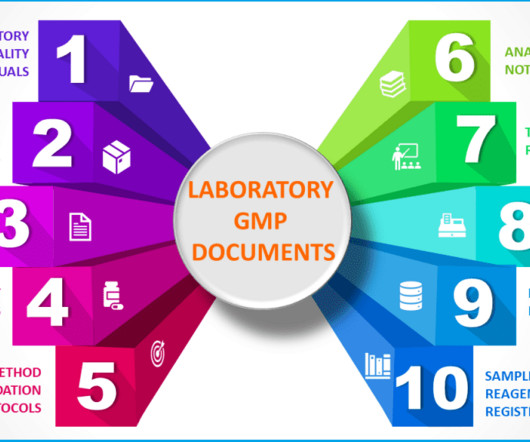
Validated test method A test method comprehensively describes all procedures used in sample analysis. Analytical test method validation guarantees that your test method is robust enough to provide evidence if your product meets the predetermined product specification or conforms to the failure of a product.








Let's personalize your content