Quality planning best practice for medical device and GMP
GMPSOP
JULY 14, 2024
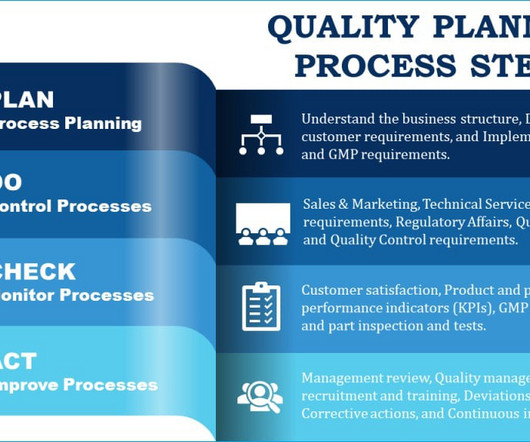
Quality planning is the blueprint to generate ultimate customer confidence and satisfaction with the products you manufacture. This article provides an in-depth look at how you, as a professional in a GMP business, can prepare quality planning documentation, especially from the perspective of a medical device manufacturing business.









Let's personalize your content