Medical Writing in Enhancing Healthcare Communication
Viseven
JULY 10, 2024
It’s like a bridge connecting the intricate world of medical science to everyday healthcare communication. As a specialized field, medical writing encompasses the creation of various documents, from regulatory submission documents to medical journal articles, that convey critical medical knowledge to diverse audiences.






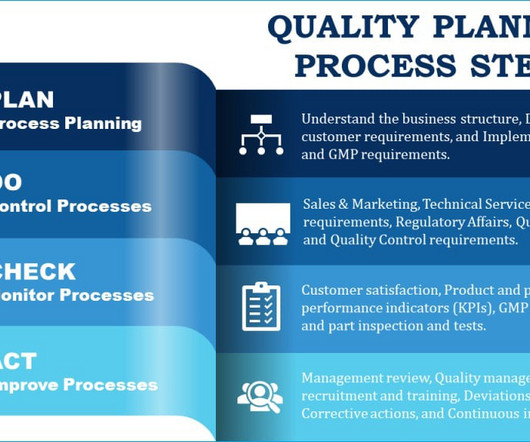


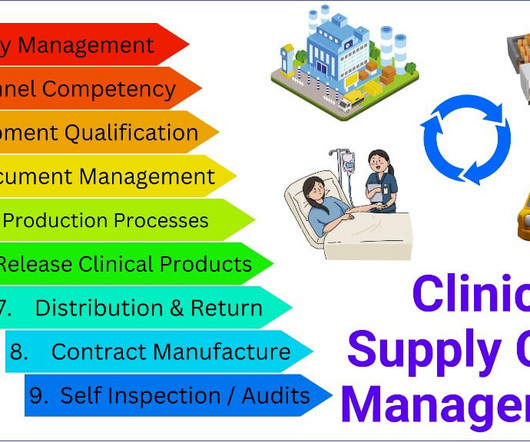
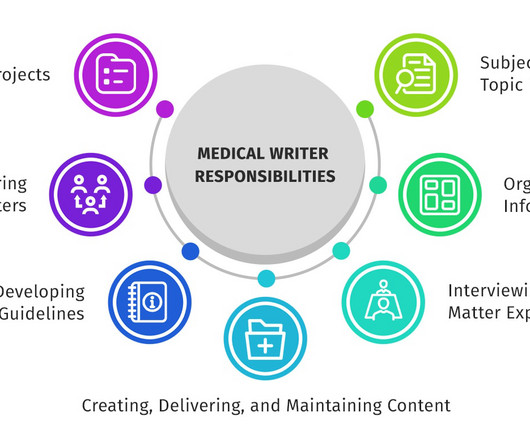
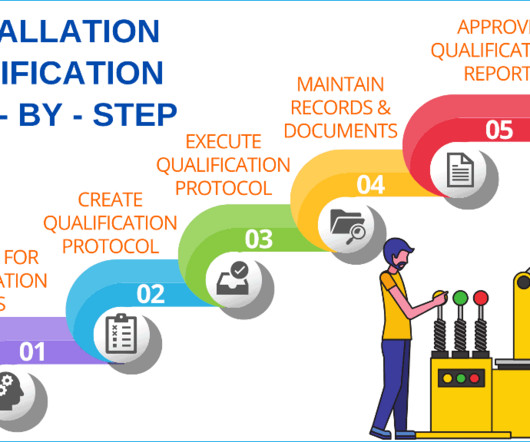


































Let's personalize your content