Fixing pulse oximeters requires federal might and possible legal action, researchers say
STAT
DECEMBER 30, 2024
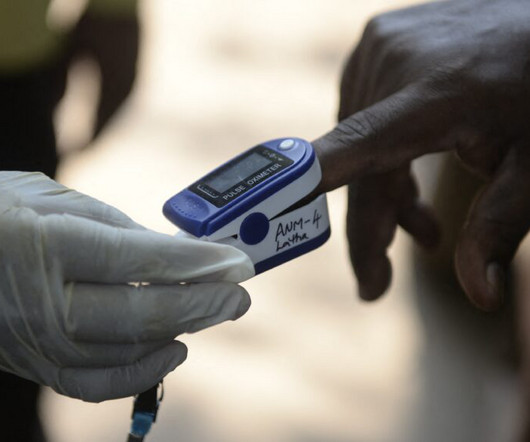
Work by device manufacturers to improve the performance of pulse oximeters on people with darker skin has progressed little since the Food and Drug Administration asked manufacturers in 2013 to voluntarily test the devices on more diverse skin tones, according to a study published Monday in JAMA. The study and a related editorial suggest clearer guidance, enforcement, and possibly legal action may be necessary to ensure the devices work well on all skin tones.






































Let's personalize your content