Radiopharmaceuticals for prostate cancer to go nuclear with $6.3 bn in sales by 2030
Express Pharma
SEPTEMBER 18, 2024
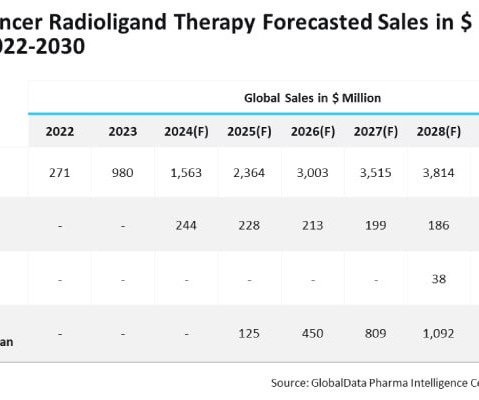
Radioligand therapies (RLTs) have received prominent attention from major pharmaceutical companies in treating prostate cancer. billion in sales by 2030, according to GlobalData. billion by 2030, far outshining Xofigo. billion by 2030. bn in sales by 2030 appeared first on Express Pharma.











Let's personalize your content