September-October 2024
Safe Biologics
NOVEMBER 14, 2024
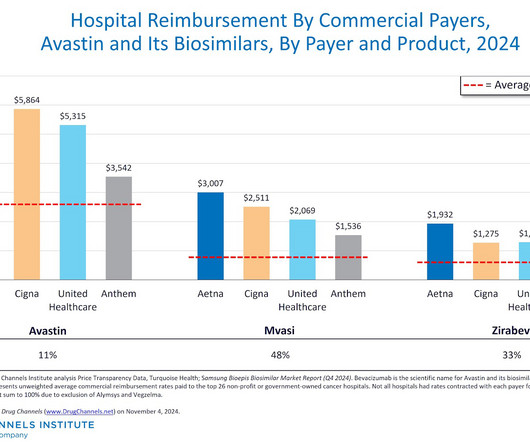
SAVE THE DATE: ASBM and GaBI to Present Webinar on the Biosimilar Red Tape Elimination Act October 31st On October 31st, ASBM and the Generics and Biosimilar Initiative will present a webinar focusing Senate Bill 2305, the Biosimilar Red Tape Elimination Act. Health policy experts will review current U.S.















































Let's personalize your content