Leuko device to remotely monitor cancer patients during chemotherapy
Pharma Times
JUNE 17, 2024
Cancer was estimated to be responsible for more than 18 million cases worldwide in 2020
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
JUNE 17, 2024
Cancer was estimated to be responsible for more than 18 million cases worldwide in 2020
Pharma Times
AUGUST 16, 2024
million diagnosed cases worldwide in 2020 This form of cancer was responsible for 1.9
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
DECEMBER 21, 2020
2020’s M&A activity hasn’t quite reached the heights of last year’s, where two pharma mega-mergers – BMS’ buyout of Celegne and AbbVie’s acquisition of Allergan – accounted for almost 40% of total M&A deal values. Here we take a look at the biggest mergers and acquisitions of 2020 and what they might mean for the companies involved.

pharmaphorum
APRIL 8, 2021
Data from trials wowed ESMO in September 2020, where Trodelvy was shown to significantly extend overall survival (OS) and improved overall response rate (ORR) and clinical benefit rate (CBR), compared with standard chemotherapy in TNBC patients with brain metastases treated with at least two therapies. billion in early 2020. .

European Pharmaceutical Review
NOVEMBER 17, 2022
Final draft guidance published by the National Institute for Health and Care Excellence (NICE), recommends Mobocertinib (Exkivity), a new therapy that helps slow cancer growth in adults with epidermal growth factor receptor ( EGFR ) exon 20+ non-small-cell lung cancer (NSCLC), who have already received platinum-based chemotherapy.

pharmaphorum
MARCH 9, 2022
There isn’t enough evidence to support the use of Merck & Co/MSD’s Keytruda in combination with chemotherapy as a first-line treatment for advanced triple negative breast cancer, according to the UK cost effectiveness watchdog NICE. Its long-term benefits are uncertain.

pharmaphorum
SEPTEMBER 21, 2020
Data from the phase 3 ASCENT study showed Trodelvy (sacituzumab govitecan) significantly extended overall survival (OS) and improved overall response rate (ORR) and clinical benefit rate (CBR), compared with standard chemotherapy in TNBC patients with brain metastases treated with at least two therapies. in the chemotherapy arm.

pharmaphorum
MAY 10, 2022
Results of the pilot in 45 patients with non-small cell lung cancer (NSCLC) treated with Tecentriq with and without chemotherapy were published in 2020. The data showed that patients as well as physicians and nursing staff found the digital tool allowed “more efficient and focused communication” as well as time savings.

European Pharmaceutical Review
NOVEMBER 6, 2023
In addition to being indicated in those with progressive disease, Mirati Therapeutics shared that the treatment is also authorised for patients with, or intolerance to, platinum-based chemotherapy and/or anti-PD-1/PD-L1 immunotherapy. Adagrasib is available for eligible NSCLC patients as a 200mg oral tablet.

Big Molecule Watch
MAY 22, 2023
According to the press release, FYLNETRA—Amneal’s third biosimilar launch in the United States since late last year—is used to treat neutropenia (low neutrophils, a type of white blood cell that fights infection) which is commonly experienced by patients undergoing chemotherapy.

pharmaphorum
JULY 20, 2022
The study compared VB-111 plus paclitaxel chemotherapy to paclitaxel given on its own, but showed no significant difference between the two groups, with PFS and OS in both coming in at just over five months and 13 months, respectively.

pharmaphorum
JUNE 11, 2021
Now, results from the ongoing TRANSFORM study show that Breyanzi was more effective than the standard second-line treatment – salvage therapy followed by high-dose chemotherapy and a stem cell transplant – at fending off disease recurrence.

pharmaphorum
OCTOBER 6, 2022
The strategy to build in cancer dates back to 2015, and has since gathered momentum with the purchase of the oncology businesses of Shire and Agios Pharma in 2018 and 2020, for $2.4 billion and $1.8 billion respectively, as well as smaller deals such as its takeover of Danish antibody specialist Symphogen.

pharmaphorum
JANUARY 18, 2021
J&J’s Janssen pharma unit got approval for Darzalex Faspro as a multiple myeloma treatment in the US last May and in Europe the following month, lending further momentum to a franchise that looks set to make more than $4 billion in sales in calendar 2020. It has already been submitted for approval in the EU.

pharmaphorum
JULY 16, 2021
The UK cost-effectiveness body first turned down Zytiga (abiraterone acetate) for this use in June 2020, but said four months later that it would reconsider its decision. That review has now concluded, and NICE has said it won’t change its mind.

pharmaphorum
OCTOBER 4, 2022
AZ and Merck’s drug was also approved as a second-line therapy after Xtandi or Pfizer’s Zytiga (abiraterone) for mCRPC in 2020, but only as a monotherapy in patients with tumours that express homologous recombination repair (HRR) gene mutation – including BRCA1/2 and ATM).

pharmaphorum
DECEMBER 14, 2021
The results come on the tail of new second-line results with Yescarta and another CD19-targeting CAR-T, Bristol-Myers Squibb’s Breyanzi (lisocabtagene maraleucel), which sought to answer the question of whether they could be an alternative to chemotherapy and a stem cell transplant – the go-to therapy for relapsed LBCL patients.
European Pharmaceutical Review
AUGUST 10, 2022
In the study ( NCT04752059 , EudraCT 2020-000981-41 ), the Austrian research team led by Matthias Preusser and Rupert Bartsch, both from the Division of Oncology, investigated the agent T-Dxd as a possible new therapeutic approach in cases where breast cancer spreads to the brain. .”

pharmaphorum
DECEMBER 9, 2022
AZ estimates that approximately 70% of breast cancer tumours are considered HR-positive and HER2-low or negative and, if first-line endocrine therapies fail, their only option currently is single-agent chemotherapy.

RX Note
MARCH 31, 2025
Patients on chemotherapy, oral corticosteroids or drugs for HIV May be susceptible to more severe infection. 2019 Clinical Study on the Effectiveness of Three Products in the Treatment of Herpes Simplex Labialis, 2020 Cold sores located in the mouth or close to the eye Outside scope of community pharmacy.

pharmaphorum
NOVEMBER 26, 2020
Y-mAbs said last month it is expecting to resubmit omburtamab for approval to the FDA in late 2020 or early 2021, and will also start clinical trials of a next-generation version of the drug before year-end in neuroblastoma as well as B7-H3-positive leptomeningeal tumours. And combination trials with chemotherapy are also planned.

Pharmaceutical Business Review
OCTOBER 23, 2024
MARGENZA, a HER2/neu receptor antagonist, received FDA approval in December 2020. It is used in combination with chemotherapy for adult patients with metastatic HER2-positive breast cancer who have previously undergone two or more anti-HER2 regimens.

pharmaphorum
APRIL 27, 2021
On Wednesday (28 April), the focus will shift to Keytruda and Tecentriq as first-line treatments for UC patients unable to be treated with cisplatin-containing chemotherapy – which got the go-ahead in 2017 and 2018, respectively. The post FDA looks at pulling speedy approvals for three cancer drugs appeared first on.

pharmaphorum
JANUARY 11, 2022
The DTx, called BNT200, is described as a first-of-its-kind DTx to treat anxiety and depression in adults with acute myeloid leukaemia (AML) who are hospitalised for a regimen of high-intensity induction chemotherapy. The DTx is an on-demand stress management intervention with content synchronised with high-intensity chemotherapy treatment.
pharmaphorum
JANUARY 26, 2021
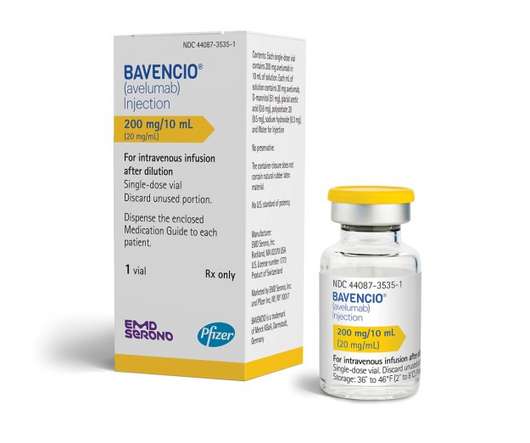
The European Commission has cleared Bavencio (avelumab) for locally advanced or metastatic urothelial carcinoma (UC) – the most common form of bladder cancer – in cases where cancer hasn’t progressed after platinum-containing chemotherapy. The green light in Europe comes six months after the FDA backed the same use for the drug in the US.

pharmaphorum
NOVEMBER 16, 2020
Phesgo has been recommended by the CHMP for use in combination with chemotherapy in early HER2-positive breast cancer – pre- and post-surgery – as well as for front-line therapy of HER2-positive breast cancer that has already spread in the body. A CHMP recommendation is usually followed by EMA approval within a few weeks. billion ($3.2

pharmaphorum
SEPTEMBER 29, 2020
As of 2018, there were over 1,100 cancer therapies in development, and as of 2020, 362 of them were cell and gene therapies. The pharmaceutical industry is one of the most scientifically innovative and competitive industries, particularly in oncology.

pharmaphorum
MAY 26, 2022
The US regulator has approved Tibsovo (ivosidenib) in combination with Bristol-Myers Squibb’s chemotherapy Vidaza (azacitidine) as a first-line therapy for IDH1-mutated acute myeloid leukaemia (AML), expanding the eligible patient population for the drug. for the Tibsovo arm and 14.9% for the control group.
pharmaphorum
JANUARY 20, 2021
billion in revenues in the first nine months of 2020 – as well as Rigel Pharma/Grifols’ recently-approved oral drug Tavalisse/Tavlesse (fostamatinib) and Amgen’s injectable Nplate (romiplostim) in the ITP indication. Sobi’s drug will have to compete with various drugs including Novartis’ Promacta (eltrombopag) – which made $1.2

Pharmaceutical Technology
JUNE 28, 2022
The treatment is intended for breast cancer patients with germline BRCA-mutated (gBRCAm), human epidermal growth factor receptor 2 (HER2)-negative who have previously received neoadjuvant or adjuvant chemotherapy. A PARP inhibitor, Lynparza is co-developed and co-marketed by Merck and AstraZeneca.

pharmaphorum
JANUARY 25, 2023
9,273,135 and 9,320,811, which cover the use of a CTLA4 inhibitor in combination with a PD-1 inhibitor and chemotherapy drug gemcitabine, respectively. Growth for the drug has picked up in the last couple of years, including approval in 2020 as a low-dose add-on to Opdivo in various liver and lung cancer settings. 1:23-cv-00079).

European Pharmaceutical Review
MAY 4, 2023
Amivantamab was the first bispecific monoclonal antibody (BsMAb) approved in the EU to treat advanced NSCLC with EGFR exon 20 insertion mutations post chemotherapy. 2020; 19(10): 2044–56. These patients face a poorer prognosis and shorter survival rates compared with lung cancer driven by more common EGFR mutations. Cancer Ther.

pharmaphorum
JULY 19, 2022
China’s Simcere Pharmaceutical has been granted approval in its home market for Cosela, a drug designed to limit the side effects of cancer chemotherapy, partnered with US biotech G1 Therapeutics. Simcere said that the number of new cancer patients requiring chemotherapy in China is expected to reach 4.2

European Pharmaceutical Review
DECEMBER 6, 2022
In July 2021, the French temporary authorisation for use (Autorisation Temporaire d’Utilisation, ATU) programme was the subject of a major reform, initially published within the 2021 healthcare plan on the 14th December 2020 (Article 78 – La Loi de financement de la sécurité sociale , FSSL). References. Available from: [link].

pharmaphorum
JULY 15, 2021
Data from the study was published in the New England Journal of Medicine in May 2020, and won tepotinib a breakthrough designation from the FDA. The MHRA said that the standard treatment of non-small cell lung cancer is chemotherapy or immunotherapy with checkpoint inhibitors if there is no targeted therapy available.

pharmaphorum
FEBRUARY 17, 2021
At an interim analysis, Lynparza (olaparib) reduced recurrence of disease compared to placebo in women with BRCA-mutated, HER2-negative breast cancer after surgery and chemotherapy to remove the tumour. million women were diagnosed with breast cancer worldwide in 2020, and BRCA mutations are found in approximately 5% of them.

Pharmaceutical Technology
SEPTEMBER 13, 2022
In 2020, Keytruda was approved for previously untreated metastatic MSI-H/dMMR CRC, with the KEYNOTE-177 study demonstrating an 8.3-month month progression-free survival (PFS) benefit over SOC chemotherapy. MSI-H/dMMR CRC accounts for 10-15% of all CRC.

Pharmaceutical Technology
JUNE 27, 2022
The transaction is focused on Epizyme’s lead asset, Tazverik (tazemetostat), a chemotherapy-free EZH2 a inhibitor. In 2020, the US Food and Drug Administration (FDA) granted accelerated approval for the drug. The company will begin an all-cash tender offer through its subsidiary to buy Epizyme’s all outstanding shares for $1.45

Pharmaceutical Technology
AUGUST 16, 2022
This was followed by a further deal for the global development and commercialisation of another ADC, datopotamab deruxtecan (DS-1062) in 2020, with a deal value of up to $6bn. months compared with patients treated with standard-of-care chemotherapy.

Pharmaceutical Technology
SEPTEMBER 26, 2022
Since 2020, palbociclib has been offered through the Cancer Drugs Fund (CDF), while additional data was obtained to address doubts on the drug’s cost efficiency and to what extent it prolongs the life expectancy of people.

European Pharmaceutical Review
DECEMBER 28, 2023
As a result, when the patient’s lymphoma relapses after treatment with chemotherapy, BV and CPIs, there are no effective treatment alternatives for these patients. Finally, Andreas looks at the prospects for antibodies that target the innate immune system in treating both haematological malignancies and solid tumours.
pharmaphorum
OCTOBER 11, 2022
The Scottish Medicines Consortium (SMC) gave the go-ahead to the regimen based on data from the KEYNOTE-775 study , which showed that the duo can increase survival time for patients and may improve quality of life by reducing the symptom burden compared to standard chemotherapy.

pharmaphorum
MARCH 14, 2022
.” One of Blue Note’s DTx products – used to treat anxiety and depression in adults with acute myeloid leukaemia (AML) who are hospitalised for a regimen of high-intensity induction chemotherapy – was awarded a breakthrough designation by the FDA earlier this year.

pharmaphorum
NOVEMBER 10, 2020
Previously treated patients with chronic lymphocytic leukaemia (CLL) in England will get a chemotherapy-free treatment option after NICE recommended NHS funding for a combination of AbbVie’s Venclyxto and Roche’s Gazyva. The decision by NICE allows for the 12-month fixed duration treatment option based on data from the phase 3 CLL14 trial.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content