What is cross contamination in pharmaceutical industry
GMPSOP
MARCH 3, 2024
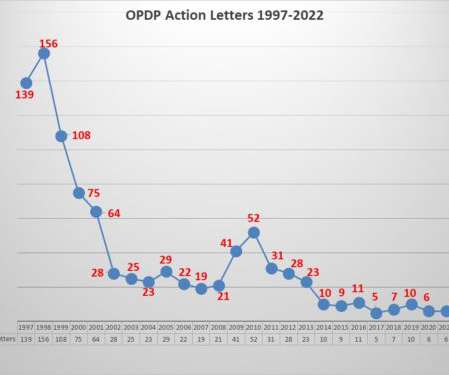
Cross contamination in the pharmaceutical industry can be described as an accidental inclusion of product of another batch or unknown foreign material into a finished batch, which was not intended or not mentioned on the label. – In 2019, the FDA publicly reprimanded 21 companies for cross-contamination.
























Let's personalize your content