Cheryl Barton
pharmaphorum
AUGUST 7, 2024
Following her senior research positions in academia and seven years with Merck & Co, she joined Dutch investment bank ABN Amro NV as a senior equity analyst to provide coverage on pan-European companies.

pharmaphorum
AUGUST 7, 2024
Following her senior research positions in academia and seven years with Merck & Co, she joined Dutch investment bank ABN Amro NV as a senior equity analyst to provide coverage on pan-European companies.
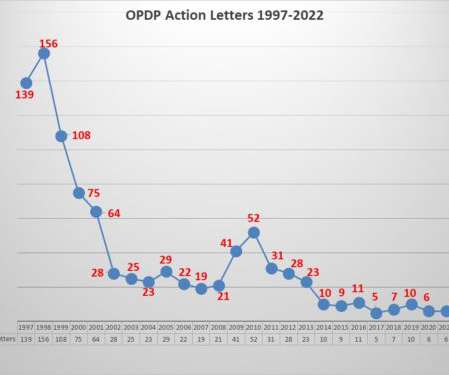
Eye on FDA
AUGUST 2, 2022
There are two means of gaining insight into the agency’s thinking about regulatory issues related to promotional communications by pharmaceutical companies; one is through the issuance of guidance documents, the other is through enforcement. But when it comes to enforcement things have changed greatly over the years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
SEPTEMBER 25, 2023
Gregory’s industrial career spans an employment history with several major pharmaceutical companies. 2002; 74(8): 1760–71. He obtained his BS in Chemistry from St Xavier College, MS in Analytical Chemistry from Governors State University and a PhD in Analytical Chemistry from Northern Illinois University. Cited 2023Feb].

pharmaphorum
DECEMBER 2, 2020
Despite this political clout, the company also tried to belay its image as a pharma monster, like many others in the industry, by spending generously on charity, donating AIDS drugs both to poor communities in the US, and to developing countries. The era of mega-mergers.

European Pharmaceutical Review
NOVEMBER 15, 2022
Lorrie Vuolo-Schuessler has been involved with ISPE and the GAMP © Community of Practice leadership since 2002 and is currently Chair of the GAMP Americas Steering Committee and a member of the GAMP Global Leadership Team. FDA, Computer Software Assurance for Production and Quality System Software – Draft Guidance. FDA, 2022. .

Express Pharma
JANUARY 12, 2024
There has been an amendment under income tax provisions to disallow such expenses incurred by pharmaceutical companies in violation of MCI regulations. – The government issues clarifications, thereby allowing ITC on such eligible business expenditure incurred on medical practitioners. . –

PharmaShots
MARCH 29, 2023
Shots: Drug patent expiry is when a patent granted to a pharmaceutical company for a particular drug expires, allowing other companies to produce and sell generic versions Like every other utility patent, pharmaceuticals also get market exclusivity of 20 years.
Let's personalize your content