5 takeaways from the Human Genome Project investigation
STAT
JULY 9, 2024
But that’s not how it turned out: One individual’s DNA accounted for the vast majority of the genome when a first draft was released in 2001. Read the rest…
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 2001 Related Topics
2001 Related Topics 
STAT
JULY 9, 2024
But that’s not how it turned out: One individual’s DNA accounted for the vast majority of the genome when a first draft was released in 2001. Read the rest…
STAT
MAY 30, 2024
at epidemic rates that have been climbing since 2001. Syphilis, one of the oldest infections known to humans, has returned to the U.S. In 2022, the last year with complete data, the highest number of infections were recorded in more than 70 years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
JUNE 5, 2024
” Here is the back story: In 2001, Gilead won U.S. For its part, the company issued a statement in which it did not admit wrongdoing and continued to maintain it has “never stopped working to improve the lives of people with HIV.”

STAT
APRIL 10, 2024
stood at 323 during the fourth quarter of last year — the highest figure reached since such data began being tracked in 2001 — underscoring growing concerns about patient harm across the country. The number of ongoing and active drug shortages in the U.S.

STAT
NOVEMBER 24, 2023
In 2001, at the age of 13, I had come to Kenya’s largest informal settlement on the outskirts of Nairobi with my sister. I had only lived in Kibera for 15 minutes when I saw a police officer and was arrested. But it would be years before I saw a real doctor. When we arrived at 3 a.m.,

STAT
AUGUST 31, 2023
The retractions come the same day that Tessier-Lavigne’s resignation as Stanford president takes effect.

STAT
JANUARY 10, 2023
Five studies co-authored by Tessier-Lavigne are now under the microscope for containing alleged altered images: a 1999 Cell study , a 2008 paper in the EMBO Journal, a 2003 Nature study, and two studies published in 2001 in Science.

STAT
MARCH 31, 2023
Indeed, academic inventors or founders contributed to more than one-quarter of all medicines approved from 2001 through 2019. Academia can be an excellent source of novel drug targets and new technologies that can enhance medicinal discovery. Yet many sponsored research collaborations that hope to tap into academia fail.

STAT
JUNE 15, 2023
The Centers for Disease Control and Prevention report examined the homicide and suicide rates among 10- to 24-year-olds from 2001 to 2021. The homicide rate for older U.S. Read the rest…

STAT
DECEMBER 12, 2022
The deal is the largest pharma merger announced this year, and it represents the most that Amgen has spent on an acquisition since its $16 billion purchase of Immunex in 2001. Amgen said Monday it would purchase Horizon Pharmaceuticals for $27.8 Continue to STAT+ to read the full story…

STAT
AUGUST 8, 2023
The study, published last week in The Lancet Psychiatry , analyzed answers that participants provided between 2001 and 2022 in response to a World Health Organization survey designed to assess the prevalence of major mental health disorders.

STAT
JANUARY 31, 2023
The program is widely considered a success and has worked well for many drugs, including the leukemia drug Gleevec , which the FDA approved in 2001 after a review period of just two-and-a-half months, based on study results that correctly predicted successful clinical outcomes. Continue to STAT+ to read the full story…

STAT
FEBRUARY 13, 2024
In 2001, Gilead secured FDA approval of tenofovir disoproxil fumarate (TDF), one of first medicines to treat HIV — a product still on the market, despite the potential side effect of causing skeletal and kidney damage.

STAT
JULY 17, 2023
The study was shut down early — and a year later, prescriptions had plummeted to nearly half what they had been in 2001. But in 2002, the therapy went into a free-fall when a landmark trial suggested treating menopause with estrogen and progesterone increased the risk of breast cancer and cardiovascular disease.

STAT
DECEMBER 12, 2022
The deal is the largest pharmaceutical merger announced this year, and it represents the most that Amgen has spent on an acquisition since its $16 billion purchase of Immunex in 2001. The The product is expected to generate $1.97 billion in 2022 and $2.26 billion in 2023, according to FactSet, an investment data firm.

STAT
APRIL 11, 2024
stood at 323 during the fourth quarter of last year — the highest figure reached since such data began being tracked in 2001 — underscoring growing concerns about patient harm across the country, STAT tells us. Unresolved The number of ongoing and active drug shortages in the U.S.

Express Pharma
FEBRUARY 14, 2025
Pillai (2013), poet-translator Madan Lal Madhu (2001), former Prime Minister Inder Kumar Gujral, Justice V.R. Dr Saxena joins an eminent group of Indians who have received the Order of Friendship , including chess grandmaster Viswanathan Anand (2018), former Member of Parliament Murli Manohar Joshi, scientist Dr A.S.

European Pharmaceutical Review
OCTOBER 19, 2023
The Investigational New Drug (IND) application for Intellia Therapeutics’ in vivo CRISPR-based candidate NTLA-2001, has been cleared as a gene editing therapy for transthyretin (ATTR) amyloidosis with cardiomyopathy (ATTR-CM) in the US. This supports NTLA-2001’s potential as a single-administration therapeutic.
Express Pharma
DECEMBER 9, 2024
The post Ichnos Glenmark Innovation presents first clinical data from phase 1 study of Trispecific TREAT Antibody, ISB 2001 appeared first on Express Pharma.

PharmaShots
APRIL 3, 2023
REGiMMUNE’s RGI-2001 is currently in P-III trial for the prophylaxis of aGVHD Ref: Businesswire | Image: Regimmune Related News:- Omega Therapeutics Entered into a Clinical Supply Agreement with Roche to Evaluate OTX-2002 for Hepatocellular Carcinoma

Outsourcing Pharma
JULY 26, 2023
Leslie Orne joined Trinity Life Sciences in 2001 and was recently appointed CEO and president of the fast-growing healthcare consultancy. She previously held the title of chief commercial officer (CCO) and president.
pharmaphorum
JUNE 28, 2021
Preliminary results from a phase 1 trial run by Regeneron and CRISPR specialist Intellia Therapeutics – co-founded by Nobel Prize winner Jennifer Doudna – showed steep reductions in a biomarker of ATTR amyloidosis disease activity with a single dose of the NTLA-2001 drug.
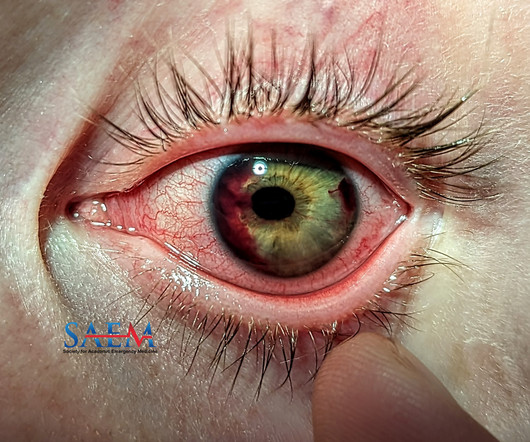
ALiEM - Pharm Pearls
SEPTEMBER 30, 2024
2001 Dec;59(12):1462-70. However, non-traumatic hyphema should prompt investigation for hematologic disorders such as Sickle cell disease. References Brandt MT, Haug RH. Traumatic hyphema: a comprehensive review. J Oral Maxillofac Surg. doi: 10.1053/joms.2001.28284. 2001.28284. PMID: 11732035. Gragg J, Blair K, Baker MB. 2022 Dec 26.

Pharmacy Times
MAY 8, 2024
Ongoing and active shortages are currently at the highest number since January 2001.

NY Times
DECEMBER 2, 2021
In 2001, U.N. estimates suggested 150 million people would be infected with H.I.V. That preceded an ambitious global campaign to curb the virus. How well did it work?

Pharmaceutical Technology
NOVEMBER 18, 2024
Intellia presented data from its Phase I study of NTLA-2001 at the 2024 American Heart Association scientific meeting in Chicago.

Pharmaceutical Technology
JULY 10, 2023
The US FDA has granted orphan drug designation (ODD) for Ichnos Sciences’ ISB 2001 to treat patients with multiple myeloma.

pharmaphorum
DECEMBER 1, 2022
Led by the MPE CEE Workgroup on Access, the report – Addressing access barriers to myeloma clinical trials in Central and Eastern Europe – evaluated the number of clinical trials held in CEE countries between 1 January 2001 and 28 September 2020. Approximately 50,000 people in Europe are diagnosed with myeloma each year.

European Pharmaceutical Review
JUNE 24, 2022
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. One of the key priorities of the EU’s strategy is to encourage innovation to solve unmet medical needs. Brussels; 2020.

pharmaphorum
NOVEMBER 18, 2020
Since being founded in 2001, Envision has delivered strong year-on-year organic growth and established leadership in medical affairs strategy, medical communications, and enterprise-wide Envision technology.

European Pharmaceutical Review
APRIL 13, 2023
For example, the relaxed attitude towards cannabis in the Netherlands is well-known and Portugal has decriminalised the possession and consumption of all illicit substances since 2001.

Pharmacy Joe
JANUARY 24, 2024
For example, if the new article is about glucose control in the ICU, you can be sure that the letter to the editor will mention other landmark trials such as 2001 article by Van Den Berghe and NICE-SUGAR.

RX Note
MARCH 31, 2025
External Links American Academy of Dermatology Association - Cold Sores Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture, 2001 Natural remedies for Herpes simplex, 2006 Randomized clinical study comparing Compeed cold sore patch to acyclovir cream 5% in the treatment of herpes simplex labialis, (..)

pharmaphorum
OCTOBER 13, 2020
Since it was founded in 2001 the company has become one of the country’s best known biotechs thanks to its approach to designing drugs based around network biology.

European Pharmaceutical Review
JANUARY 25, 2024
Proposal for a Directive of the European Parliament and of the Council on the Union code relating to medicinal products for human use, and repealing Directive 2001/83/EC and Directive 2009/35/EC.” References European Commission, (26 April 2023). Article 63 EMA (29 January 2020). Article 63 Euractiv (26 September 2023).

pharmaphorum
JANUARY 6, 2022
H3B-8800 – now renamed RVT-2001 – will be taken in a slightly different direction by Roivant, which said it plans to develop it as a therapy for transfusion-dependent anaemia in patients with lower-risk MDS, according to the company.

FDA Law Blog: Biosimilars
MAY 3, 2023
Before joining HP&M in 2001, Mr. Houck conducted numerous scheduled cyclic and targeted inspections and investigations as a DEA Diversion Investigator in the field for ten years. Registrants are far from powerless when DEA investigators inspect their controlled substance operations.

pharmaphorum
DECEMBER 14, 2020
Since the beginning of her career in 2001, Elena has partnered with various stakeholders, such as patients, healthcare professionals and life-science companies, to create impact for modern marketing activities. She can be contacted via elena.pirofalo@healthwareinternational.com.

European Pharmaceutical Review
JULY 4, 2024
11 The THR pathway was created with the purpose of establishing a simplified registration procedure for all traditional herbal medicines that do not fulfil the requirements for the MA (strict) pathway under Directive 2001/83/EC. 10,12 The THR scheme is also limited to products used for oral or external use and/or inhalation. 2012/1916).

Pharmaceutical Technology
JULY 18, 2022
The latest contract comes after previous agreements between the parties for seasonal influenza and pandemic vaccines dating back to 2001. The company intends to supply both vaccines from its 230,000ft² Sainte-Foy facility in Quebec. .

RX Note
JUNE 13, 2023
External Links Clinical Inertia and Outpatient Medical Errors Clinical inertia, 2001 Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review, 2020

Pharma Leaders
MAY 12, 2023
Founded in 2001 and headquartered in Guelph, Ontario, Canada, Nutrasource is a global contract research organisation (CRO) in the pharmaceutical and nutraceutical businesses. The company will initially buy a 60% interest in Nutrasource while having the right to pick the remaining 40% holding in 2026.

Pharmaceutical Business Review
MAY 12, 2023
Founded in 2001 and headquartered in Guelph, Ontario, Canada, Nutrasource is a global contract research organisation (CRO) in the pharmaceutical and nutraceutical businesses. The company will initially buy a 60% interest in Nutrasource while having the right to pick the remaining 40% holding in 2026.

Express Pharma
MARCH 5, 2025
Where is the goodwill we earned when the Father of Generics, our own Dr Yusuf Hamied paved the way for generics way back in the year 2001? Looking at the recent incidents in the Indian pharma industry, are we applying our knowledge ethically?
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content